Colon Cancer Growth and Dissemination Relies upon Thrombin, Stromal PAR-1, and Fibrinogen
- PMID: 26238780
- PMCID: PMC4809049
- DOI: 10.1158/0008-5472.CAN-15-0964
Colon Cancer Growth and Dissemination Relies upon Thrombin, Stromal PAR-1, and Fibrinogen
Abstract
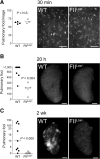
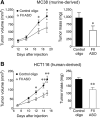
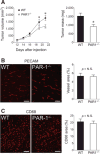
Thrombin-mediated proteolysis is a major determinant of metastasis, but is not universally important for primary tumor growth. Here, we report that colorectal adenocarcinoma represents one important exception whereby thrombin-mediated functions support both primary tumor growth and metastasis. In contrast with studies of multiple nongastrointestinal cancers, we found that the growth of primary tumors formed by murine and human colon cancer cells was reduced in mice by genetic or pharmacologic reduction of circulating prothrombin. Reduced prothrombin expression was associated with lower mitotic indices and invasion of surrounding tissue. Mechanistic investigations revealed that thrombin-driven colonic adenocarcinoma growth relied upon at least two targets of thrombin-mediated proteolysis, protease-activated receptor-1 (PAR-1) expressed by stromal cells and the extracellular matrix protein, fibrinogen. Colonic adenocarcinoma growth was reduced in PAR-1-deficient mice, implicating stromal cell-associated PAR-1 as one thrombin target important for tumor outgrowth. Furthermore, tumor growth was dramatically impeded in fibrinogen-deficient mice, offering the first direct evidence of a critical functional role for fibrinogen in malignant tumor growth. Tumors harvested from fibrinogen-deficient mice displayed a relative reduction in cell proliferative indices, as well as increased tumor necrosis and decreased tumor vascular density. Collectively, our findings established a functional role for thrombin and its targets PAR-1 and fibrinogen in the pathogenesis of colonic adenocarcinoma, supporting tumor growth as well as local invasion and metastasis.
©2015 American Association for Cancer Research.
Conflict of interest statement
B.P. Monia is Senior Vice President and has ownership interest (including patents) in Isis Pharmaceuticals. No potential conflicts of interest were disclosed by the other authors.
Figures


References
-
- Palumbo JS. Mechanisms linking tumor cell-associated procoagulant function to tumor dissemination. Semin Thromb Hemost. 2008;34:154–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

