Hepatitis B Virus and Hepatitis D Virus Entry, Species Specificity, and Tissue Tropism
- PMID: 26238794
- PMCID: PMC4526719
- DOI: 10.1101/cshperspect.a021378
Hepatitis B Virus and Hepatitis D Virus Entry, Species Specificity, and Tissue Tropism
Abstract
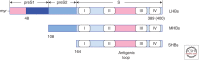
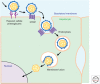
Entry of hepatitis B (HBV) and hepatitis D viruses (HDV) into a host cell represents the initial step of infection. This process requires multiple steps, including the low-affinity attachment of the virus to the cell surface, followed by high-affinity attachment to specific receptor(s), and subsequent endocytosis-mediated internalization. Within the viral envelope, the preS1 region is involved in receptor binding. Recently, sodium taurocholate cotransporting polypeptide (NTCP) has been identified as an entry receptor of HBV and HDV by affinity purification using a preS1 peptide. NTCP is mainly or exclusively expressed in the liver, and this membrane protein is at least one of the factors determining the narrow species specificity and hepatotropism of HBV and HDV. However, there are likely other factors that mediate the species and tissue tropism of HBV. This review summarizes the current understanding of the mechanisms of HBV/HDV entry.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

References
-
- Bremer CM, Sominskaya I, Skrastina D, Pumpens P, El Wahed AA, Beutling U, Frank R, Fritz HJ, Hunsmann G, Gerlich WH, et al. 2011. N-terminal myristoylation-dependent masking of neutralizing epitopes in the preS1 attachment site of hepatitis B virus. J Hepatol 55: 29–37. - PubMed
-
- Bruss V, Hagelstein J, Gerhardt E, Galle PR. 1996. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology 218: 396–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
