Effects of brain‑derived neurotrophic factor and neurotrophin‑3 on the neuronal differentiation of rat adipose‑derived stem cells
- PMID: 26239042
- PMCID: PMC4581787
- DOI: 10.3892/mmr.2015.4099
Effects of brain‑derived neurotrophic factor and neurotrophin‑3 on the neuronal differentiation of rat adipose‑derived stem cells
Abstract
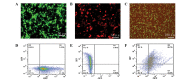
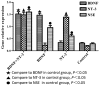
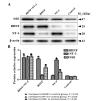
Tissue engineering is a promising method that may be used to treat spinal cord injury (SCI). The underlying repair mechanism of tissue engineering involves the stable secretion of neurotrophins from seed cells, which eventually differentiate into neurons; therefore, the selection of appropriate seed cells, which stably secrete neurotrophins that easily differentiate into neurons requires investigation. Adipose‑derived stem cells (ADSCs), which are adult SCs, are advantageous due to convenience sampling and easy expansion; therefore, ADSCs are currently the most popular type of seed cell. Brain‑derived neurotrophic factor (BDNF) and neurotrophin‑3 (NT‑3) possess superior properties, when compared with other neurotrophic factors, in the maintenance of neuronal survival and promotion of SC differentiation into neurons. The present study used two lentiviruses, which specifically express BDNF and NT‑3 [Lenti‑BDNF‑green fluorescent protein (GFP), Lenti‑NT‑3‑red fluorescent protein (RFP)], to transfect third‑generation ADSCs. Three types of seed cell were obtained: i) Seed cells overexpressing BDNF (ADSC/Lenti‑BDNF‑GFP); ii) seed cells overexpressing NT‑3 (ADSC/Lenti‑NT‑3‑RFP); and iii) seed cells overexpressing BDNF and NT‑3 (ADSC/Lenti‑BDNF‑GFP and NT‑3‑RFP). The transfected cells were then induced to differentiate into neurons and were divided into a further four groups: i) The BDNF and NT‑3 co‑overexpression group; ii) the BDNF overexpression group; iii) the NT‑3 overexpression group; and iv) the control group, which consisted of untransfected ADSCs. The results of the present study demonstrate that BDNF and NT‑3 expression was higher 10 days after induction, as detected by reverse transcription‑quantitative polymerase chain reaction (RT‑qPCR) and western blotting. Neuron‑specific enolase is a neuronal marker, the expression of which was highest in the BDNF and NT‑3 co‑overexpression group, followed by the BDNF overexpression group and then by the NT‑3 overexpression group. The lowest expression levels of NSE were detected in the control group, as determined by RT‑qPCR, western blotting and immunofluorescent staining. These results indicate that BDNF and NT‑3 exert a synergistic effect, which may promote the neuronal differentiation of ADSCs. The present study provides a solid theoretical foundation for future experiments regarding the use of tissue engineering technology for the treatment of SCI.
Figures



References
-
- Kanno H, Pressman Y, Moody A, Berg R, Muir EM, Rogers JH, Ozawa H, Itoi E, Pearse DD, Bunge MB. Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. J Neurosci. 2014;34:1838–1855. doi: 10.1523/JNEUROSCI.2661-13.2014. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

