Anti-leukemic activity of axitinib against cells harboring the BCR-ABL T315I point mutation
- PMID: 26239229
- PMCID: PMC4523922
- DOI: 10.1186/s13045-015-0190-9
Anti-leukemic activity of axitinib against cells harboring the BCR-ABL T315I point mutation
Abstract
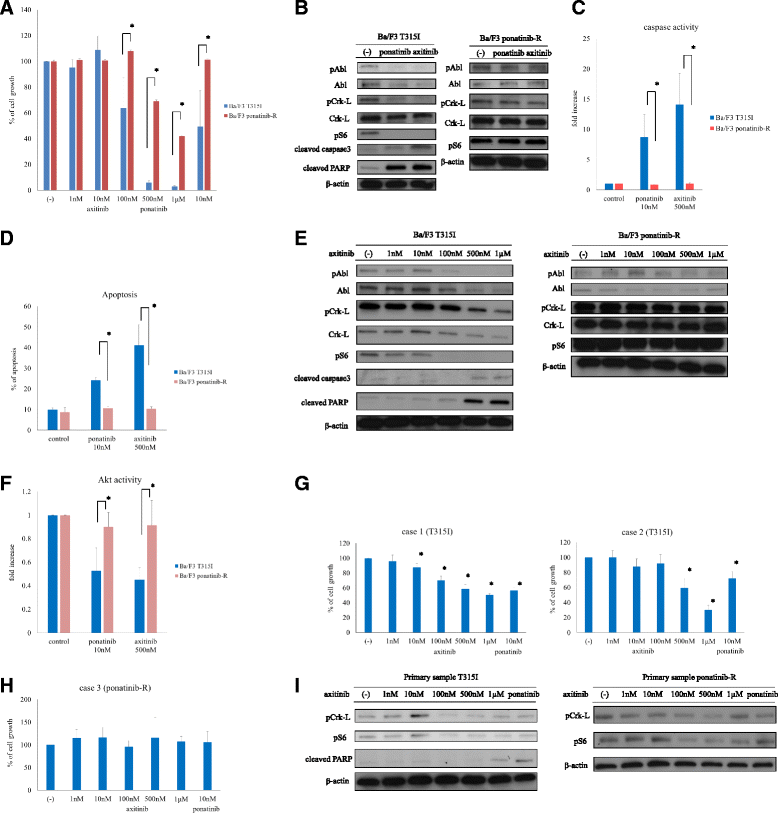
The BCR-ABL; breakpoint cluster region-Abelson point mutation T315I is resistant to ABL tyrosine kinase inhibitors. However, axitinib, a vascular endothelial growth factor receptor inhibitor, is effective against this mutation. In this study, we investigated axitinib activity against ponatinib-resistant cells and found that axitinib inhibited cellular growth and apoptosis in Ba/F3 T315I-mutant cells and T315I-mutant primary samples, but not in ponatinib-resistant Ba/F3 cells and primary samples. Thus, an alternative strategy may be required to improve the prognosis of Philadelphia-chromosome-positive leukemia patients harboring BCR-ABL point mutations.
Figures
Similar articles
-
Axitinib effectively inhibits BCR-ABL1(T315I) with a distinct binding conformation.Nature. 2015 Mar 5;519(7541):102-5. doi: 10.1038/nature14119. Epub 2015 Feb 9. Nature. 2015. PMID: 25686603
-
Combining the ABL1 kinase inhibitor ponatinib and the histone deacetylase inhibitor vorinostat: a potential treatment for BCR-ABL-positive leukemia.PLoS One. 2014 Feb 28;9(2):e89080. doi: 10.1371/journal.pone.0089080. eCollection 2014. PLoS One. 2014. PMID: 24586514 Free PMC article.
-
PF-114, a potent and selective inhibitor of native and mutated BCR/ABL is active against Philadelphia chromosome-positive (Ph+) leukemias harboring the T315I mutation.Leukemia. 2015 May;29(5):1104-14. doi: 10.1038/leu.2014.326. Epub 2014 Nov 14. Leukemia. 2015. PMID: 25394714
-
[Is AP24534 (Ponatinib) the next treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia?].Bull Cancer. 2011 Jul;98(7):761-7. doi: 10.1684/bdc.2011.1390. Bull Cancer. 2011. PMID: 21700550 Review. French.
-
BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review.Leuk Res. 2010 Oct;34(10):1255-68. doi: 10.1016/j.leukres.2010.04.016. Leuk Res. 2010. PMID: 20537386 Review.
Cited by
-
Axitinib in Ponatinib-Resistant B-Cell Acute Lymphoblastic Leukemia Harboring a T315L Mutation.Int J Mol Sci. 2020 Dec 20;21(24):9724. doi: 10.3390/ijms21249724. Int J Mol Sci. 2020. PMID: 33419251 Free PMC article.
-
Axitinib and sorafenib are potent in tyrosine kinase inhibitor resistant chronic myeloid leukemia cells.Cell Commun Signal. 2016 Feb 24;14:6. doi: 10.1186/s12964-016-0129-y. Cell Commun Signal. 2016. PMID: 26912052 Free PMC article.
-
Effect of axitinib regulating the pathological blood-brain barrier functional recovery for glioblastoma therapeutics.CNS Neurosci Ther. 2022 Mar;28(3):411-421. doi: 10.1111/cns.13788. Epub 2021 Dec 29. CNS Neurosci Ther. 2022. PMID: 34967104 Free PMC article.
-
Anti-Proliferative Effect of Allium senescens L. Extract in Human T-Cell Acute Lymphocytic Leukemia Cells.Molecules. 2020 Dec 23;26(1):35. doi: 10.3390/molecules26010035. Molecules. 2020. PMID: 33374788 Free PMC article.
-
Best Practices in Chronic Myeloid Leukemia Monitoring and Management.Oncologist. 2016 May;21(5):626-33. doi: 10.1634/theoncologist.2015-0337. Epub 2016 Mar 31. Oncologist. 2016. PMID: 27032870 Free PMC article. Review.
References
-
- Kurzrock R, Shtalrid M, Talpaz M, Kloetzer WS, Gutterman JU. Expression of c-abl in Philadelphia-positive acute myelogenous leukemia. Blood. 1987;70:1584–1588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

