A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models
- PMID: 26239362
- PMCID: PMC4753781
- DOI: 10.1038/ncomms8939
A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models
Abstract
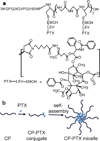
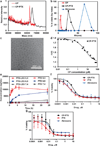
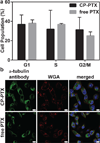
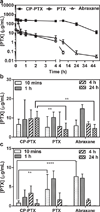
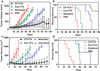
Packaging clinically relevant hydrophobic drugs into a self-assembled nanoparticle can improve their aqueous solubility, plasma half-life, tumour-specific uptake and therapeutic potential. To this end, here we conjugated paclitaxel (PTX) to recombinant chimeric polypeptides (CPs) that spontaneously self-assemble into ∼60 nm near-monodisperse nanoparticles that increased the systemic exposure of PTX by sevenfold compared with free drug and twofold compared with the Food and Drug Administration-approved taxane nanoformulation (Abraxane). The tumour uptake of the CP-PTX nanoparticle was fivefold greater than free drug and twofold greater than Abraxane. In a murine cancer model of human triple-negative breast cancer and prostate cancer, CP-PTX induced near-complete tumour regression after a single dose in both tumour models, whereas at the same dose, no mice treated with Abraxane survived for >80 days (breast) and 60 days (prostate), respectively. These results show that a molecularly engineered nanoparticle with precisely engineered design features outperforms Abraxane, the current gold standard for PTX delivery.
Conflict of interest statement
Figures
References
-
- Lee CC, MacKay JA, Frechet JMJ, Szoka FC. Designing dendrimers for biological applications. Nature Biotechnol. 2005;23:1517–1526. - PubMed
-
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

