Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque
- PMID: 26239536
- PMCID: PMC4623970
- DOI: 10.1002/ana.24497
Clinical and pathological insights into the dynamic nature of the white matter multiple sclerosis plaque
Abstract
Objective: An extensive analysis of white matter plaques in a large sample of multiple sclerosis (MS) autopsies provides insights into the dynamic nature of MS pathology.
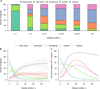
Methods: One hundred twenty MS cases (1,220 tissue blocks) were included. Plaque types were classified according to demyelinating activity based on stringent criteria. Early active, late active, smoldering, inactive, and shadow plaques were distinguished. A total of 2,476 MS white matter plaques were identified. Plaque type distribution was analyzed in relation to clinical data.
Results: Active plaques were most often found in early disease, whereas at later stages, smoldering, inactive, and shadow plaques predominated. The presence of early active plaques rapidly declined with disease duration. Plaque type distribution differed significantly by clinical course. The majority of plaques in acute monophasic and relapsing-remitting MS (RRMS) were active. Among secondary progressive MS (SPMS) cases with attacks, all plaque types could be distinguished including active plaques, in contrast to SPMS without attacks, in which inactive plaques predominated. Smoldering plaques were frequently and almost exclusively found in progressive MS. At 47 years of age, an equilibrium was observed between active and inactive plaques, whereas smoldering plaques began to peak. Men displayed a higher proportion of smoldering plaques.
Interpretation: Disease duration, clinical course, age, and gender contribute to the dynamic nature of white matter MS pathology. Active MS plaques predominate in acute and early RRMS and are the likely substrate of clinical attacks. Progressive MS transitions to an accumulation of smoldering plaques characterized by microglial activation and slow expansion of pre-existing plaques. Whether current MS therapeutics impact this pathological driver of disease progression remains uncertain.
© 2015 American Neurological Association.
Figures




References
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343(13):938–952. - PubMed
-
- Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol. 2012;8(11):647–656. - PubMed
-
- Popescu BF, Lucchinetti CF. Pathology of demyelinating diseases. Annu Rev Pathol. 2012;7:185–217. - PubMed
-
- Kutzelnigg A, Lassmann H. Cortical lesions and brain atrophy in MS. J Neurol Sci. 2005;233(1–2):55–59. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

