Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation
- PMID: 26239654
- PMCID: PMC4571836
- DOI: 10.1093/cvr/cvv205
Gene therapy for cardiovascular disease: advances in vector development, targeting, and delivery for clinical translation
Abstract
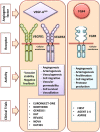
Gene therapy is a promising modality for the treatment of inherited and acquired cardiovascular diseases. The identification of the molecular pathways involved in the pathophysiology of heart failure and other associated cardiac diseases led to encouraging preclinical gene therapy studies in small and large animal models. However, the initial clinical results yielded only modest or no improvement in clinical endpoints. The presence of neutralizing antibodies and cellular immune responses directed against the viral vector and/or the gene-modified cells, the insufficient gene expression levels, and the limited gene transduction efficiencies accounted for the overall limited clinical improvements. Nevertheless, further improvements of the gene delivery technology and a better understanding of the underlying biology fostered renewed interest in gene therapy for heart failure. In particular, improved vectors based on emerging cardiotropic serotypes of the adeno-associated viral vector (AAV) are particularly well suited to coax expression of therapeutic genes in the heart. This led to new clinical trials based on the delivery of the sarcoplasmic reticulum Ca(2+)-ATPase protein (SERCA2a). Though the first clinical results were encouraging, a recent Phase IIb trial did not confirm the beneficial clinical outcomes that were initially reported. New approaches based on S100A1 and adenylate cyclase 6 are also being considered for clinical applications. Emerging paradigms based on the use of miRNA regulation or CRISPR/Cas9-based genome engineering open new therapeutic perspectives for treating cardiovascular diseases by gene therapy. Nevertheless, the continuous improvement of cardiac gene delivery is needed to allow the use of safer and more effective vector doses, ultimately bringing gene therapy for heart failure one step closer to reality.
Keywords: Adeno-associated viral vector; Adenylate cyclase; CRISPR; Cardiovascular disease; Clinical trials; Gene therapy; Heart failure; S100A1; SERCA2a; miRNA.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures

Similar articles
-
Advances in gene therapy for heart failure.Discov Med. 2015 Apr;19(105):285-91. Discov Med. 2015. PMID: 25977191 Review.
-
Benefit of SERCA2a gene transfer to vascular endothelial and smooth muscle cells: a new aspect in therapy of cardiovascular diseases.Curr Vasc Pharmacol. 2013 Jul;11(4):465-79. doi: 10.2174/1570161111311040010. Curr Vasc Pharmacol. 2013. PMID: 23905641 Free PMC article. Review.
-
The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases.Nat Clin Pract Cardiovasc Med. 2008 Sep;5(9):554-65. doi: 10.1038/ncpcardio1301. Epub 2008 Jul 29. Nat Clin Pract Cardiovasc Med. 2008. PMID: 18665137 Review.
-
Gene therapy for heart failure.J Cardiol. 2015 Sep;66(3):195-200. doi: 10.1016/j.jjcc.2015.02.006. Epub 2015 Mar 25. J Cardiol. 2015. PMID: 25818479 Review.
-
Adeno-associated virus-mediated gene therapy in cardiovascular disease.Curr Opin Cardiol. 2015 May;30(3):228-34. doi: 10.1097/HCO.0000000000000159. Curr Opin Cardiol. 2015. PMID: 25783685 Free PMC article. Review.
Cited by
-
Gene Therapy and Cardiovascular Diseases.Adv Exp Med Biol. 2023;1396:235-254. doi: 10.1007/978-981-19-5642-3_16. Adv Exp Med Biol. 2023. PMID: 36454471
-
Determination of Anti-Adeno-Associated Viral Vector Neutralizing Antibodies in Patients With Heart Failure in the Cardiovascular Foundation of Colombia (ANVIAS): Study Protocol.JMIR Res Protoc. 2016 Jun 9;5(2):e102. doi: 10.2196/resprot.5535. JMIR Res Protoc. 2016. PMID: 27282359 Free PMC article.
-
Targeting and delivery of microRNA-targeting antisense oligonucleotides in cardiovascular diseases.Atherosclerosis. 2023 Jun;374:44-54. doi: 10.1016/j.atherosclerosis.2022.12.003. Epub 2022 Dec 19. Atherosclerosis. 2023. PMID: 36577600 Free PMC article. Review.
-
Current Landscape of Gene Therapy for the Treatment of Cardiovascular Disorders.Curr Gene Ther. 2024;24(5):356-376. doi: 10.2174/0115665232268840231222035423. Curr Gene Ther. 2024. PMID: 38288826 Review.
-
Bioengineering Technologies for Cardiac Regenerative Medicine.Front Bioeng Biotechnol. 2021 Jun 3;9:681705. doi: 10.3389/fbioe.2021.681705. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34150737 Free PMC article. Review.
References
-
- Bleumink GS, Knetsch AM, Sturkenboom MCJM, Straus SMJM, Hofman A, Deckers JW, Witteman JCM, Stricker BHC. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J 2004;25:1614–1619. - PubMed
-
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557–573. - PubMed
-
- Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet 2011;12:316–328. - PubMed
-
- Yerevanian A, Yerevanian A, Hajjar RJ. Progress in gene therapy for heart failure. J Cardiovasc Pharmacol 2014;63:95–106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

