Mitochondrial Glutathione in Diabetic Nephropathy
- PMID: 26239684
- PMCID: PMC4519798
- DOI: 10.3390/jcm4071428
Mitochondrial Glutathione in Diabetic Nephropathy
Abstract
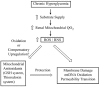
Although there are many etiologies for diabetic nephropathy (DN), one common characteristic of all cases involves mitochondrial oxidative stress and consequent bioenergetic dysfunction. As the predominant low-molecular-weight, intramitochondrial thiol reductant, the mitochondrial glutathione (mtGSH) pool plays important roles in how this organelle adapts to the chronic hyperglycemia and redox imbalances associated with DN. This review will summarize information about the processes by which this important GSH pool is regulated and how manipulation of these processes can affect mitochondrial and cellular function in the renal proximal tubule. Mitochondria in renal proximal tubular (PT) cells do not appear to synthesize GSH de novo but obtain it by transport from the cytoplasm. Two inner membrane organic anion carriers, the dicarboxylate carrier (DIC; Slc25a10) and 2-oxoglutarate carrier (OGC; Slc25a11) are responsible for this transport. Genetic modulation of DIC or OGC expression in vitro in PT cells from diabetic rats can alter mitochondrial function and susceptibility of renal PT cells to oxidants, with overexpression leading to reversion of bioenergetic conditions to a non-diabetic state and protection of cells from injury. These findings support the mtGSH carriers as potential therapeutic targets to correct the underlying metabolic disturbance in DN.
Keywords: diabetic nephropathy; gene expression; glutathione; mitochondria; oxidative stress; transport.
Figures




References
-
- Geiss L.S., Herman W.H., Goldschmid M.G., DeStefano F., Eberhardt M.S., Ford E.S., German R.R., Newman J.M., Olson D.R., Sepe S.J. Surveillance for diabetes mellitus: United States, 1980–1989. MMWR CDC Surveill. Summ. 1993;42:1–20. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

