Schwann Cells Increase Prostate and Pancreatic Tumor Cell Invasion Using Laminin Binding A6 Integrin
- PMID: 26239765
- PMCID: PMC4809241
- DOI: 10.1002/jcb.25300
Schwann Cells Increase Prostate and Pancreatic Tumor Cell Invasion Using Laminin Binding A6 Integrin
Abstract
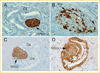
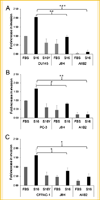
Human pancreatic and prostate cancers metastasize along nerve axons during perineural invasion. The extracellular matrix laminin class of proteins is an abundant component of both myelinated and non-myelinated nerves. Analysis of human pancreatic and prostate tissue revealed both perineural and endoneural invasion with Schwann cells surrounded or disrupted by tumor, respectively. Tumor and nerve cell co-culture conditions were used to determine if myelinating or non-myelinating Schwann cell (S16 and S16Y, respectively) phenotype was equally likely to promote integrin-dependent cancer cell invasion and migration on laminin. Conditioned medium from S16 cells increased tumor cell (DU145, PC3, and CFPAC1) invasion into laminin approximately 1.3-2.0 fold compared to fetal bovine serum (FBS) treated cells. Integrin function (e.g., ITGA6p formation) increased up to 1.5 fold in prostate (DU145, PC3, RWPE-1) and pancreatic (CFPAC1) cells, and invasion was dependent on ITGA6p formation and ITGB1 as determined by function-blocking antibodies. In contrast, conditioned medium isolated from S16Y cells (non-myelinating phenotype) decreased constitutive levels of ITGA6p in the tumor cells by 50% compared to untreated cells and decreased ITGA6p formation 3.0 fold compared to S16 treated cells. Flow cytometry and western blot analysis revealed loss of ITGA6p formation as reversible and independent of overall loss of ITGA6 expression. These results suggest that the myelinating phenotype of Schwann cells within the tumor microenvironment increased integrin-dependent tumor invasion on laminin.
Keywords: A6B1; LAMININ; METASTASIS; MICROENVIRONMENT; PERINEURAL INVASION; SCHWANN CELLS.
© 2015 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest: None.
Figures



References
-
- Bapat AA, Hostetter G, Von Hoff DD, Han H. Perineural invasion and associated pain in pancreatic cancer. Nat Rev Cancer. 2011;11(10):695–707. - PubMed
-
- Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Muller MW, Giese NA, Friess H, Schafer KH. Neural invasion in pancreatic cancer: A mutual tropism between neurons and cancer cells. Biochemi Biophys Res Commun. 2008;374(3):442–447. - PubMed
-
- Chernousov MA, Yu WM, Chen ZL, Carey DJ, Strickland S. Regulation of Schwann cell function by the extracellular matrix. Glia. 2008;56(14):1498–1507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

