Opposite and Coordinated Rotation of Amphitrichous Flagella Governs Oriented Swimming and Reversals in a Magnetotactic Spirillum
- PMID: 26240070
- PMCID: PMC4573727
- DOI: 10.1128/JB.00172-15
Opposite and Coordinated Rotation of Amphitrichous Flagella Governs Oriented Swimming and Reversals in a Magnetotactic Spirillum
Abstract
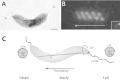
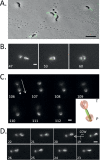
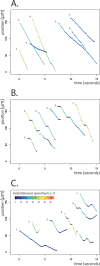
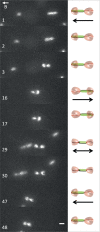
Current knowledge regarding the mechanism that governs flagellar motor rotation in response to environmental stimuli stems mainly from the study of monotrichous and peritrichous bacteria. Little is known about how two polar flagella, one at each cell pole of the so-called amphitrichous bacterium, are coordinated to steer the swimming. Here we fluorescently labeled the flagella of Magnetospirillum magneticum AMB-1 cells and took advantage of the magnetically controllable swimming of this bacterium to investigate flagellar rotation in moving cells. We identified three motility behaviors (runs, tumbles, and reversals) and two characteristic fluorescence patterns likely corresponding to flagella rotating in opposite directions. Each AMB-1 locomotion mode was systematically associated with particular flagellar patterns at the poles which led us to conclude that, while cell runs are allowed by the asymmetrical rotation of flagellar motors, their symmetrical rotation triggers cell tumbling. Our observations point toward a precise coordination of the two flagellar motors which can be temporarily unsynchronized during tumbling.
Importance: Motility is essential for bacteria to search for optimal niches and survive. Many bacteria use one or several flagella to explore their environment. The mechanism by which bipolarly flagellated cells coordinate flagellar rotation is poorly understood. We took advantage of the genetic amenability and magnetically controlled swimming of the spirillum-shaped magnetotactic bacterium Magnetospirillum magneticum AMB-1 to correlate cell motion with flagellar rotation. We found that asymmetric rotation of the flagella (counterclockwise at the lagging pole and clockwise at the leading pole) enables cell runs whereas symmetric rotation triggers cell tumbling. Taking into consideration similar observations in spirochetes, bacteria possessing bipolar ribbons of periplasmic flagella, we propose a conserved motility paradigm for spirillum-shaped bipolarly flagellated bacteria.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

