Demonstration of Lignin-to-Peroxidase Direct Electron Transfer: A TRANSIENT-STATE KINETICS, DIRECTED MUTAGENESIS, EPR, AND NMR STUDY
- PMID: 26240145
- PMCID: PMC4645588
- DOI: 10.1074/jbc.M115.665919
Demonstration of Lignin-to-Peroxidase Direct Electron Transfer: A TRANSIENT-STATE KINETICS, DIRECTED MUTAGENESIS, EPR, AND NMR STUDY
Erratum in
-
Demonstration of lignin-to-peroxidase direct electron transfer. A TRANSIENT-STATE KINETICS, DIRECTED MUTAGENESIS, EPR AND NMR STUDY.J Biol Chem. 2015 Dec 18;290(51):30268. doi: 10.1074/jbc.A115.665919. J Biol Chem. 2015. PMID: 26684341 Free PMC article. No abstract available.
Abstract
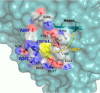
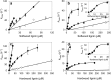
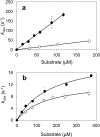
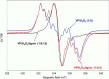
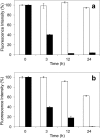
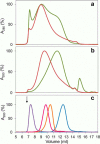
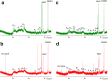
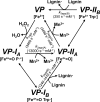
Versatile peroxidase (VP) is a high redox-potential peroxidase of biotechnological interest that is able to oxidize phenolic and non-phenolic aromatics, Mn(2+), and different dyes. The ability of VP from Pleurotus eryngii to oxidize water-soluble lignins (softwood and hardwood lignosulfonates) is demonstrated here by a combination of directed mutagenesis and spectroscopic techniques, among others. In addition, direct electron transfer between the peroxidase and the lignin macromolecule was kinetically characterized using stopped-flow spectrophotometry. VP variants were used to show that this reaction strongly depends on the presence of a solvent-exposed tryptophan residue (Trp-164). Moreover, the tryptophanyl radical detected by EPR spectroscopy of H2O2-activated VP (being absent from the W164S variant) was identified as catalytically active because it was reduced during lignosulfonate oxidation, resulting in the appearance of a lignin radical. The decrease of lignin fluorescence (excitation at 355 nm/emission at 400 nm) during VP treatment under steady-state conditions was accompanied by a decrease of the lignin (aromatic nuclei and side chains) signals in one-dimensional and two-dimensional NMR spectra, confirming the ligninolytic capabilities of the enzyme. Simultaneously, size-exclusion chromatography showed an increase of the molecular mass of the modified residual lignin, especially for the (low molecular mass) hardwood lignosulfonate, revealing that the oxidation products tend to recondense during the VP treatment. Finally, mutagenesis of selected residues neighboring Trp-164 resulted in improved apparent second-order rate constants for lignosulfonate reactions, revealing that changes in its protein environment (modifying the net negative charge and/or substrate accessibility/binding) can modulate the reactivity of the catalytic tryptophan.
Keywords: HSQC NMR; electron paramagnetic resonance (EPR); enzyme kinetics; lignin degradation; lignosulfonate; nuclear magnetic resonance (NMR); peroxidase; transient-state kinetics; tryptophanyl radical; versatile peroxidase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Protein radicals in fungal versatile peroxidase: catalytic tryptophan radical in both compound I and compound II and studies on W164Y, W164H, and W164S variants.J Biol Chem. 2009 Mar 20;284(12):7986-94. doi: 10.1074/jbc.M808069200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19158088 Free PMC article.
-
Role of surface tryptophan for peroxidase oxidation of nonphenolic lignin.Biotechnol Biofuels. 2016 Sep 17;9:198. doi: 10.1186/s13068-016-0615-x. eCollection 2016. Biotechnol Biofuels. 2016. PMID: 28616078 Free PMC article.
-
Versatile peroxidase oxidation of high redox potential aromatic compounds: site-directed mutagenesis, spectroscopic and crystallographic investigation of three long-range electron transfer pathways.J Mol Biol. 2005 Nov 25;354(2):385-402. doi: 10.1016/j.jmb.2005.09.047. Epub 2005 Oct 3. J Mol Biol. 2005. PMID: 16246366
-
Substrate oxidation sites in versatile peroxidase and other basidiomycete peroxidases.J Exp Bot. 2009;60(2):441-52. doi: 10.1093/jxb/ern261. Epub 2008 Nov 5. J Exp Bot. 2009. PMID: 18987391 Review.
-
A new versatile peroxidase from Pleurotus.Biochem Soc Trans. 2001 May;29(Pt 2):116-22. doi: 10.1042/0300-5127:0290116. Biochem Soc Trans. 2001. PMID: 11356138 Review.
Cited by
-
Peroxidase evolution in white-rot fungi follows wood lignin evolution in plants.Proc Natl Acad Sci U S A. 2019 Sep 3;116(36):17900-17905. doi: 10.1073/pnas.1905040116. Epub 2019 Aug 19. Proc Natl Acad Sci U S A. 2019. PMID: 31427536 Free PMC article.
-
Fungal lignin peroxidase does not produce the veratryl alcohol cation radical as a diffusible ligninolytic oxidant.J Biol Chem. 2018 Mar 30;293(13):4702-4712. doi: 10.1074/jbc.RA117.001153. Epub 2018 Feb 9. J Biol Chem. 2018. PMID: 29462790 Free PMC article.
-
Ligninolytic enzymes: Versatile biocatalysts for the elimination of endocrine-disrupting chemicals in wastewater.Microbiologyopen. 2018 Dec;7(6):e00722. doi: 10.1002/mbo3.722. Epub 2018 Oct 17. Microbiologyopen. 2018. PMID: 30328673 Free PMC article. Review.
-
Evolutionary convergence in lignin-degrading enzymes.Proc Natl Acad Sci U S A. 2018 Jun 19;115(25):6428-6433. doi: 10.1073/pnas.1802555115. Epub 2018 Jun 4. Proc Natl Acad Sci U S A. 2018. PMID: 29866821 Free PMC article.
-
Structure-function characterization of two enzymes from novel subfamilies of manganese peroxidases secreted by the lignocellulose-degrading Agaricales fungi Agrocybe pediades and Cyathus striatus.Biotechnol Biofuels Bioprod. 2024 Jun 1;17(1):74. doi: 10.1186/s13068-024-02517-1. Biotechnol Biofuels Bioprod. 2024. PMID: 38824538 Free PMC article.
References
-
- Ragauskas A. J., Beckham G. T., Biddy M. J., Chandra R., Chen F., Davis M. F., Davison B. H., Dixon R. A., Gilna P., Keller M., Langan P., Naskar A. K., Saddler J. N., Tschaplinski T. J., Tuskan G. A., Wyman C. E. (2014) Lignin valorization: improving lignin processing in the biorefinery. Science 344, 1246843. - PubMed
-
- Himmel M. E., Ding S. Y., Johnson D. K., Adney W. S., Nimlos M. R., Brady J. W., Foust T. D. (2007) Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science 315, 804–807 - PubMed
-
- Martínez A. T., Ruiz-Dueñas F. J., Martínez M. J., Del Río J. C., Gutiérrez A. (2009) Enzymatic delignification of plant cell wall: from nature to mill. Curr. Opin. Biotechnol. 20, 348–357 - PubMed
-
- Ahmad M., Roberts J. N., Hardiman E. M., Singh R., Eltis L. D., Bugg T. D. H. (2011) Identification of DypB from Rhodococcus jostii RHA1 as a lignin peroxidase. Biochemistry 50, 5096–5107 - PubMed

