Vinculin phosphorylation at residues Y100 and Y1065 is required for cellular force transmission
- PMID: 26240176
- PMCID: PMC4582403
- DOI: 10.1242/jcs.172031
Vinculin phosphorylation at residues Y100 and Y1065 is required for cellular force transmission
Abstract
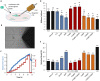
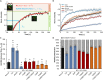
The focal adhesion protein vinculin connects the actin cytoskeleton, through talin and integrins, with the extracellular matrix. Vinculin consists of a globular head and tail domain, which undergo conformational changes from a closed auto-inhibited conformation in the cytoplasm to an open conformation in focal adhesions. Src-mediated phosphorylation has been suggested to regulate this conformational switch. To explore the role of phosphorylation in vinculin activation, we used knock-out mouse embryonic fibroblasts re-expressing different vinculin mutants in traction microscopy, magnetic tweezer microrheology, FRAP and actin-binding assays. Compared to cells expressing wild-type or constitutively active vinculin, we found reduced tractions, cytoskeletal stiffness, adhesion strength, and increased vinculin dynamics in cells expressing constitutively inactive vinculin or vinculin where Src-mediated phosphorylation was blocked by replacing tyrosine at position 100 and/or 1065 with a non-phosphorylatable phenylalanine residue. Replacing tyrosine residues with phospho-mimicking glutamic acid residues restored cellular tractions, stiffness and adhesion strength, as well as vinculin dynamics, and facilitated vinculin-actin binding. These data demonstrate that Src-mediated phosphorylation is necessary for vinculin activation, and that phosphorylation controls cytoskeletal mechanics by regulating force transmission between the actin cytoskeleton and focal adhesion proteins.
Keywords: Actin pulldown; Cell stiffness; FRAP; Focal adhesion; Mechanotransduction; Traction; Tyrosine phosphorylation; Vinculin.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Bays J. L., Peng X., Tolbert C. E., Guilluy C., Angell A. E., Pan Y., Superfine R., Burridge K. and DeMali K. A. (2014). Vinculin phosphorylation differentially regulates mechanotransduction at cell-cell and cell-matrix adhesions. J. Cell Biol. 205, 251-263. 10.1083/jcb.201309092 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

