Accessing Real-Life Episodic Information from Minutes versus Hours Earlier Modulates Hippocampal and High-Order Cortical Dynamics
- PMID: 26240179
- PMCID: PMC4961013
- DOI: 10.1093/cercor/bhv155
Accessing Real-Life Episodic Information from Minutes versus Hours Earlier Modulates Hippocampal and High-Order Cortical Dynamics
Abstract
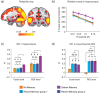
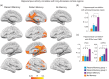
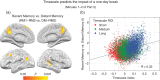
It is well known that formation of new episodic memories depends on hippocampus, but in real-life settings (e.g., conversation), hippocampal amnesics can utilize information from several minutes earlier. What neural systems outside hippocampus might support this minutes-long retention? In this study, subjects viewed an audiovisual movie continuously for 25 min; another group viewed the movie in 2 parts separated by a 1-day delay. Understanding Part 2 depended on retrieving information from Part 1, and thus hippocampus was required in the day-delay condition. But is hippocampus equally recruited to access the same information from minutes earlier? We show that accessing memories from a few minutes prior elicited less interaction between hippocampus and default mode network (DMN) cortical regions than accessing day-old memories of identical events, suggesting that recent information was available with less reliance on hippocampal retrieval. Moreover, the 2 groups evinced reliable but distinct DMN activity timecourses, reflecting differences in information carried in these regions when Part 1 was recent versus distant. The timecourses converged after 4 min, suggesting a time frame over which the continuous-viewing group may have relied less on hippocampal retrieval. We propose that cortical default mode regions can intrinsically retain real-life episodic information for several minutes.
Keywords: declarative memory; functional connectivity; intersubject correlation; naturalistic; timescales.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- Aggleton JP. 2012. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev. 36:1579–1596. - PubMed
-
- Aggleton JP, Shaw C, Gaffan EA. 1992. The performance of postencephalitic amnesic subjects on two behavioural tests of memory: concurrent discrimination learning and delayed matching-to-sample. Cortex. 28:359–372. - PubMed
-
- Ames DL, Honey CJ, Chow MA, Todorov A, Hasson U. 2014. Contextual alignment of cognitive and neural dynamics. J Cogn Neurosci. 27:655–664. - PubMed
-
- Baddeley A, Wilson BA. 2002. Prose recall and amnesia: implications for the structure of working memory. Neuropsychologia. 40:1737–1743. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

