Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies
- PMID: 26240231
- PMCID: PMC4554747
- DOI: 10.1200/JCO.2014.60.2383
Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin Determined by Digital Gene Expression in Formalin-Fixed Paraffin-Embedded Tissue Biopsies
Abstract
Purpose: To evaluate the prognostic impact of cell-of-origin (COO) subgroups, assigned using the recently described gene expression-based Lymph2Cx assay in comparison with International Prognostic Index (IPI) score and MYC/BCL2 coexpression status (dual expressers).
Patients and methods: Reproducibility of COO assignment using the Lymph2Cx assay was tested employing repeated sampling within tumor biopsies and changes in reagent lots. The assay was then applied to pretreatment formalin-fixed paraffin-embedded tissue (FFPET) biopsies from 344 patients with de novo diffuse large B-cell lymphoma (DLBCL) uniformly treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) at the British Columbia Cancer Agency. MYC and BCL2 protein expression was assessed using immunohistochemistry on tissue microarrays.
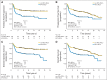
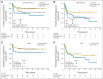
Results: The Lymph2Cx assay provided concordant COO calls in 96% of 49 repeatedly sampled tumor biopsies and in 100% of 83 FFPET biopsies tested across reagent lots. Critically, no frank misclassification (activated B-cell-like DLBCL to germinal center B-cell-like DLBCL or vice versa) was observed. Patients with activated B-cell-like DLBCL had significantly inferior outcomes compared with patients with germinal center B-cell-like DLBCL (log-rank P < .001 for time to progression, progression-free survival, disease-specific survival, and overall survival). In pairwise multivariable analyses, COO was associated with outcomes independent of IPI score and MYC/BCL2 immunohistochemistry. The prognostic significance of COO was particularly evident in patients with intermediate IPI scores and the non-MYC-positive/BCL2-positive subgroup (log-rank P < .001 for time to progression).
Conclusion: Assignment of DLBCL COO by the Lymph2Cx assay using FFPET biopsies identifies patient groups with significantly different outcomes after R-CHOP, independent of IPI score and MYC/BCL2 dual expression.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Prognostic Significance of Diffuse Large B-Cell Lymphoma Cell of Origin: Seeing the Forest and the Trees.J Clin Oncol. 2015 Sep 10;33(26):2835-6. doi: 10.1200/JCO.2015.61.9288. Epub 2015 Aug 10. J Clin Oncol. 2015. PMID: 26261249 No abstract available.
References
-
- Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (ed 4) Lyon, France: IARC Press; 2008.
-
- Alizadeh AA, Elsen MB, Davis ER, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. - PubMed
-
- Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

