Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer
- PMID: 26240233
- PMCID: PMC4554746
- DOI: 10.1200/JCO.2015.61.4776
Use and Effectiveness of Intraperitoneal Chemotherapy for Treatment of Ovarian Cancer
Abstract
Purpose: A 2006 randomized trial demonstrated a 16-month survival benefit with intraperitoneal and intravenous (IP/IV) chemotherapy administered to patients who had ovarian cancer, compared with IV chemotherapy alone, but more treatment-related toxicities. The objective of this study was to examine the use and effectiveness of IP/IV chemotherapy in clinical practice.
Patients and methods: Prospective cohort study of 823 women with stage III, optimally cytoreduced ovarian cancer diagnosed at six National Comprehensive Cancer Network institutions. We examined IP/IV chemotherapy use in all patients diagnosed between 2003 and 2012 (N = 823), and overall survival and treatment-related toxicities with Cox regression and logistic regression, respectively, in a propensity score-matched sample (n = 402) of patients diagnosed from 2006 to 2012, excluding trial participants, to minimize selection bias.
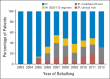
Results: Use of IP/IV chemotherapy increased from 0% to 33% between 2003 and 2006, increased to 50% from 2007 to 2008, and plateaued thereafter. Between 2006 and 2012, adoption of IP/IV chemotherapy varied by institution from 4% to 67% (P < .001) and 43% of patients received modified IP/IV regimens at treatment initiation. In the propensity score-matched sample, IP/IV chemotherapy was associated with significantly improved overall survival (3-year overall survival, 81% v 71%; hazard ratio, 0.68; 95% CI, 0.47 to 0.99), compared with IV chemotherapy, but also more frequent alterations in chemotherapy delivery route (adjusted rates discontinuation or change, 20.4% v 10.0%; adjusted odds ratio, 2.83; 95% CI, 1.47 to 5.47).
Conclusion: Although the use of IP/IV chemotherapy increased significantly at National Comprehensive Cancer Network centers between 2003 and 2012, fewer than 50% of eligible patients received it. Increasing IP/IV chemotherapy use in clinical practice may be an important and underused strategy to improve ovarian cancer outcomes.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures


Comment in
-
Effective ovarian cancer treatment is underused, US study finds.BMJ. 2015 Aug 6;351:h4291. doi: 10.1136/bmj.h4291. BMJ. 2015. PMID: 26251364 No abstract available.
-
Potential Simpson's Paradox in Multicenter Study of Intraperitoneal Chemotherapy for Ovarian Cancer.J Clin Oncol. 2016 Mar 20;34(9):1016. doi: 10.1200/JCO.2015.64.4542. Epub 2016 Jan 11. J Clin Oncol. 2016. PMID: 26755513 No abstract available.
-
Reply to G.B. Holt.J Clin Oncol. 2016 Mar 20;34(9):1016-7. doi: 10.1200/JCO.2015.65.3782. Epub 2016 Jan 11. J Clin Oncol. 2016. PMID: 26755516 No abstract available.
References
-
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. - PubMed
-
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. - PubMed
-
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: An intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. - PubMed
-
- Peppercorn JM, Weeks JC, Cook EF, et al. Comparison of outcomes in cancer patients treated within and outside clinical trials: Conceptual framework and structured review. Lancet. 2004;363:263–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

