Immunosuppression-Independent Role of Regulatory T Cells against Hypertension-Driven Renal Dysfunctions
- PMID: 26240279
- PMCID: PMC4573708
- DOI: 10.1128/MCB.00518-15
Immunosuppression-Independent Role of Regulatory T Cells against Hypertension-Driven Renal Dysfunctions
Abstract
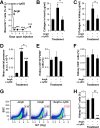
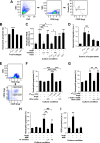
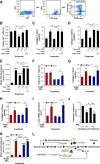
Hypertension-associated cardiorenal diseases represent one of the heaviest burdens for current health systems. In addition to hemodynamic damage, recent results have revealed that hematopoietic cells contribute to the development of these diseases by generating proinflammatory and profibrotic environments in the heart and kidney. However, the cell subtypes involved remain poorly characterized. Here we report that CD39(+) regulatory T (TREG) cells utilize an immunosuppression-independent mechanism to counteract renal and possibly cardiac damage during angiotensin II (AngII)-dependent hypertension. This mechanism relies on the direct apoptosis of tissue-resident neutrophils by the ecto-ATP diphosphohydrolase activity of CD39. In agreement with this, experimental and genetic alterations in TREG/TH cell ratios have a direct impact on tissue-resident neutrophil numbers, cardiomyocyte hypertrophy, cardiorenal fibrosis, and, to a lesser extent, arterial pressure elevation during AngII-driven hypertension. These results indicate that TREG cells constitute a first protective barrier against hypertension-driven tissue fibrosis and, in addition, suggest new therapeutic avenues to prevent hypertension-linked cardiorenal diseases.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
