Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma
- PMID: 26240291
- PMCID: PMC4539580
- DOI: 10.1158/1078-0432.CCR-14-2467
Identification of a Three-Biomarker Panel in Urine for Early Detection of Pancreatic Adenocarcinoma
Abstract
Purpose: Noninvasive biomarkers for early detection of pancreatic ductal adenocarcinoma (PDAC) are currently not available. Here, we aimed to identify a set of urine proteins able to distinguish patients with early-stage PDAC from healthy individuals.
Experimental design: Proteomes of 18 urine samples from healthy controls, chronic pancreatitis, and patients with PDAC (six/group) were assayed using GeLC/MS/MS analysis. The selected biomarkers were subsequently validated with ELISA assays using multiple logistic regression applied to a training dataset in a multicenter cohort comprising 488 urine samples.
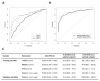
Results: LYVE-1, REG1A, and TFF1 were selected as candidate biomarkers. When comparing PDAC (n = 192) with healthy (n = 87) urine specimens, the resulting areas under the receiver-operating characteristic curves (AUC) of the panel were 0.89 [95% confidence interval (CI), 0.84-0.94] in the training (70% of the data) and 0.92 (95% CI, 0.86-0.98) in the validation (30% of the data) datasets. When comparing PDAC stage I-II (n = 71) with healthy urine specimens, the panel achieved AUCs of 0.90 (95% CI, 0.84-0.96) and 0.93 (95% CI, 0.84-1.00) in the training and validation datasets, respectively. In PDAC stage I-II and healthy samples with matching plasma CA19.9, the panel achieved a higher AUC of 0.97 (95% CI, 0.94-0.99) than CA19.9 (AUC = 0.88; 95% CI, 0.81-0.95, P = 0.005). Adding plasma CA19.9 to the panel increased the AUC from 0.97 (95% CI, 0.94-0.99) to 0.99 (95% CI, 0.97-1.00, P = 0.04), but did not improve the comparison of stage I-IIA PDAC (n = 17) with healthy urine.
Conclusions: We have established a novel, three-protein biomarker panel that is able to detect patients with early-stage pancreatic cancer in urine specimens.
©2015 American Association for Cancer Research.
Figures

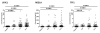


References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24:792–800. - PubMed
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014;74:2913–21. - PubMed
-
- Furukawa H, Okada S, Saisho H, Ariyama J, Karasawa E, Nakaizumi A, et al. Clinicopathologic features of small pancreatic adenocarcinoma. A collective study. Cancer. 1996;78:986–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

