Nucleotides regulate the mechanical hierarchy between subdomains of the nucleotide binding domain of the Hsp70 chaperone DnaK
- PMID: 26240360
- PMCID: PMC4547238
- DOI: 10.1073/pnas.1504625112
Nucleotides regulate the mechanical hierarchy between subdomains of the nucleotide binding domain of the Hsp70 chaperone DnaK
Abstract
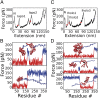
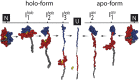
The regulation of protein function through ligand-induced conformational changes is crucial for many signal transduction processes. The binding of a ligand alters the delicate energy balance within the protein structure, eventually leading to such conformational changes. In this study, we elucidate the energetic and mechanical changes within the subdomains of the nucleotide binding domain (NBD) of the heat shock protein of 70 kDa (Hsp70) chaperone DnaK upon nucleotide binding. In an integrated approach using single molecule optical tweezer experiments, loop insertions, and steered coarse-grained molecular simulations, we find that the C-terminal helix of the NBD is the major determinant of mechanical stability, acting as a glue between the two lobes. After helix unraveling, the relative stability of the two separated lobes is regulated by ATP/ADP binding. We find that the nucleotide stays strongly bound to lobe II, thus reversing the mechanical hierarchy between the two lobes. Our results offer general insights into the nucleotide-induced signal transduction within members of the actin/sugar kinase superfamily.
Keywords: ATPase; elasticity; force; laser trapping; protein extension.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Janovjak H, Sapra KT, Kedrov A, Muller DJ. From valleys to ridges: Exploring the dynamic energy landscape of single membrane proteins. Chemphyschem. 2008;9(7):954–966. - PubMed
-
- Kedrov A, Ziegler C, Muller DJ. Differentiating ligand and inhibitor interactions of a single antiporter. J Mol Biol. 2006;362(5):925–932. - PubMed
-
- Manibog K, Li H, Rakshit S, Sivasankar S. Resolving the molecular mechanism of cadherin catch bond formation. Nat Commun. 2014;5:3941. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

