Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands
- PMID: 26240369
- PMCID: PMC4547250
- DOI: 10.1073/pnas.1505529112
Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands
Abstract
Primary aldosteronism (PA) represents the most common cause of secondary hypertension, but little is known regarding its adrenal cellular origins. Recently, aldosterone-producing cell clusters (APCCs) with high expression of aldosterone synthase (CYP11B2) were found in both normal and PA adrenal tissue. PA-causing aldosterone-producing adenomas (APAs) harbor mutations in genes encoding ion channels/pumps that alter intracellular calcium homeostasis and cause renin-independent aldosterone production through increased CYP11B2 expression. Herein, we hypothesized that APCCs have APA-related aldosterone-stimulating somatic gene mutations. APCCs were studied in 42 normal adrenals from kidney donors. To clarify APCC molecular characteristics, we used microarrays to compare the APCC transcriptome with conventional adrenocortical zones [zona glomerulosa (ZG), zona fasciculata, and zona reticularis]. The APCC transcriptome was most similar to ZG but with an enhanced capacity to produce aldosterone. To determine if APCCs harbored APA-related mutations, we performed targeted next generation sequencing of DNA from 23 APCCs and adjacent normal adrenal tissue isolated from both formalin-fixed, paraffin-embedded, and frozen tissues. Known aldosterone driver mutations were identified in 8 of 23 (35%) APCCs, including mutations in calcium channel, voltage-dependent, L-type, α1D-subunit (CACNA1D; 6 of 23 APCCs) and ATPase, Na(+)/(K+) transporting, α1-polypeptide (ATP1A1; 2 of 23 APCCs), which were not observed in the adjacent normal adrenal tissue. Overall, we show three major findings: (i) APCCs are common in normal adrenals, (ii) APCCs harbor somatic mutations known to cause excess aldosterone production, and (iii) the mutation spectrum of aldosterone-driving mutations is different in APCCs from that seen in APA. These results provide molecular support for APCC as a precursor of PA.
Keywords: adrenal; aldosterone; aldosterone-producing cell cluster; primary aldosteronism; somatic mutations.
Conflict of interest statement
Conflict of interest statement: S.A.T has a separate sponsored research agreement with Compendia Bioscience/Life Technologies. No part of the study described herein was supported by Compendia Bioscience/Life Technologies, and they had no role in the data collection, interpretation, or analysis, and did not participate in the study design or decision to submit for publication.
Figures

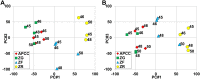


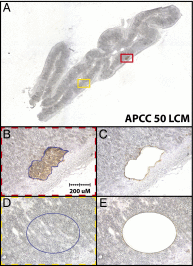
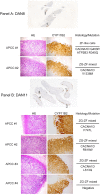



Comment in
-
Adrenal gland: Aldosterone-producing mutations in normal adrenal glands.Nat Rev Endocrinol. 2015 Oct;11(10):567. doi: 10.1038/nrendo.2015.145. Epub 2015 Aug 25. Nat Rev Endocrinol. 2015. PMID: 26303598 No abstract available.
Similar articles
-
Characterization of Aldosterone-producing Cell Cluster (APCC) at Single-cell Resolution.J Clin Endocrinol Metab. 2022 Aug 18;107(9):2439-2448. doi: 10.1210/clinem/dgac394. J Clin Endocrinol Metab. 2022. PMID: 35796577 Free PMC article.
-
Cellular and Genetic Causes of Idiopathic Hyperaldosteronism.Hypertension. 2018 Oct;72(4):874-880. doi: 10.1161/HYPERTENSIONAHA.118.11086. Hypertension. 2018. PMID: 30354720 Free PMC article.
-
Mass Spectrometry Imaging Establishes 2 Distinct Metabolic Phenotypes of Aldosterone-Producing Cell Clusters in Primary Aldosteronism.Hypertension. 2020 Mar;75(3):634-644. doi: 10.1161/HYPERTENSIONAHA.119.14041. Epub 2020 Jan 20. Hypertension. 2020. PMID: 31957522
-
The Potential Role of Aldosterone-Producing Cell Clusters in Adrenal Disease.Horm Metab Res. 2020 Jun;52(6):427-434. doi: 10.1055/a-1128-0421. Epub 2020 Mar 30. Horm Metab Res. 2020. PMID: 32227317 Free PMC article. Review.
-
Immunohistochemistry of aldosterone synthase leads the way to the pathogenesis of primary aldosteronism.Mol Cell Endocrinol. 2017 Feb 5;441:124-133. doi: 10.1016/j.mce.2016.10.014. Epub 2016 Oct 14. Mol Cell Endocrinol. 2017. PMID: 27751767 Free PMC article. Review.
Cited by
-
Aldosterone-Regulating Receptors and Aldosterone-Driver Somatic Mutations.Front Endocrinol (Lausanne). 2021 Mar 16;12:644382. doi: 10.3389/fendo.2021.644382. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33796077 Free PMC article.
-
Intracellular Molecular Differences in Aldosterone- Compared to Cortisol-Secreting Adrenal Cortical Adenomas.Front Endocrinol (Lausanne). 2016 Jun 27;7:75. doi: 10.3389/fendo.2016.00075. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27445978 Free PMC article. Review.
-
Functional imaging with 11C-metomidate PET for subtype diagnosis in primary aldosteronism.Eur J Endocrinol. 2020 Dec;183(6):539-550. doi: 10.1530/EJE-20-0532. Eur J Endocrinol. 2020. PMID: 33055298 Free PMC article.
-
Functional Zonation of the Adult Mammalian Adrenal Cortex.Front Neurosci. 2016 Jun 15;10:238. doi: 10.3389/fnins.2016.00238. eCollection 2016. Front Neurosci. 2016. PMID: 27378832 Free PMC article. Review.
-
Disorganized Steroidogenesis in Adrenocortical Carcinoma, a Case Study.Endocr Pathol. 2017 Mar;28(1):27-35. doi: 10.1007/s12022-016-9441-8. Endocr Pathol. 2017. PMID: 27430645 Free PMC article.
References
-
- Hannemann A, Wallaschofski H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies--a review of the current literature. Horm Metab Res. 2012;44(3):157–162. - PubMed
-
- Husebye ES, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275(2):104–115. - PubMed
-
- Chao CT, et al. Diagnosis and management of primary aldosteronism: An updated review. Ann Med. 2013;45(4):375–383. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

