Using perturbations to identify the brain circuits underlying active vision
- PMID: 26240420
- PMCID: PMC4528817
- DOI: 10.1098/rstb.2014.0205
Using perturbations to identify the brain circuits underlying active vision
Abstract
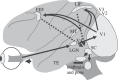
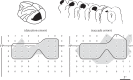
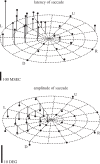
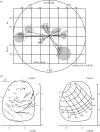
The visual and oculomotor systems in the brain have been studied extensively in the primate. Together, they can be regarded as a single brain system that underlies active vision--the normal vision that begins with visual processing in the retina and extends through the brain to the generation of eye movement by the brainstem. The system is probably one of the most thoroughly studied brain systems in the primate, and it offers an ideal opportunity to evaluate the advantages and disadvantages of the series of perturbation techniques that have been used to study it. The perturbations have been critical in moving from correlations between neuronal activity and behaviour closer to a causal relation between neuronal activity and behaviour. The same perturbation techniques have also been used to tease out neuronal circuits that are related to active vision that in turn are driving behaviour. The evolution of perturbation techniques includes ablation of both cortical and subcortical targets, punctate chemical lesions, reversible inactivations, electrical stimulation, and finally the expanding optogenetic techniques. The evolution of perturbation techniques has supported progressively stronger conclusions about what neuronal circuits in the brain underlie active vision and how the circuits themselves might be organized.
Keywords: behaviour; brain-circuits; inactivations; lesions; monkeys; optogenetics.
Figures


References
-
- Kuffler SW. 1953. Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 16, 37–68. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

