Functionalized α-Helical Peptide Hydrogels for Neural Tissue Engineering
- PMID: 26240838
- PMCID: PMC4517957
- DOI: 10.1021/acsbiomaterials.5b00051
Functionalized α-Helical Peptide Hydrogels for Neural Tissue Engineering
Erratum in
-
Correction to "Functionalized α-Helical Peptide Hydrogels for Neural Tissue Engineering".ACS Biomater Sci Eng. 2025 Oct 13;11(10):6302-6303. doi: 10.1021/acsbiomaterials.5c00792. Epub 2025 Aug 31. ACS Biomater Sci Eng. 2025. PMID: 40886131 No abstract available.
Abstract
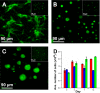
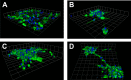
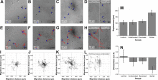
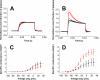
Trauma to the central and peripheral nervous systems often lead to serious morbidity. Current surgical methods for repairing or replacing such damage have limitations. Tissue engineering offers a potential alternative. Here we show that functionalized α-helical-peptide hydrogels can be used to induce attachment, migration, proliferation and differentiation of murine embryonic neural stem cells (NSCs). Specifically, compared with undecorated gels, those functionalized with Arg-Gly-Asp-Ser (RGDS) peptides increase the proliferative activity of NSCs; promote their directional migration; induce differentiation, with increased expression of microtubule-associated protein-2, and a low expression of glial fibrillary acidic protein; and lead to the formation of larger neurospheres. Electrophysiological measurements from NSCs grown in RGDS-decorated gels indicate developmental progress toward mature neuron-like behavior. Our data indicate that these functional peptide hydrogels may go some way toward overcoming the limitations of current approaches to nerve-tissue repair.
Keywords: RGD peptide; hydrogel; nerve tissue engineering; peptide; self-assembly; stem cell.
Figures

References
-
- Chen J.; Lee H. J.; Jakovcevski I.; Shah R.; Bhagat N.; Loers G.; Liu H.-Y.; Meiners S.; Taschenberger G.; Kügler S.; Irintchev A.; Schachner M. The Extracellular Matrix Glycoprotein Tenascin-C is Beneficial for Spinal Cord Regeneration. Mol. Ther. 2010, 18, 1769–1777 10.1038/mt.2010.133. - DOI - PMC - PubMed
Grants and funding
- NC/C014103/1/NC3RS_/National Centre for the Replacement, Refinement and Reduction of Animals in Research/United Kingdom
- PB-PG-1013-32058/DH_/Department of Health/United Kingdom
- BB/H01716X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L01386X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- G1100623/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
