Progesterone and Overlooked Endocrine Pathways in Breast Cancer Pathogenesis
- PMID: 26241069
- PMCID: PMC4588833
- DOI: 10.1210/en.2015-1392
Progesterone and Overlooked Endocrine Pathways in Breast Cancer Pathogenesis
Abstract
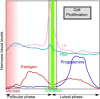
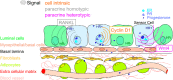
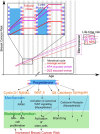
Worldwide, breast cancer incidence has been increasing for decades. Exposure to reproductive hormones, as occurs with recurrent menstrual cycles, affects breast cancer risk, and can promote disease progression. Exogenous hormones and endocrine disruptors have also been implicated in increasing breast cancer incidence. Numerous in vitro studies with hormone-receptor-positive cell lines have provided insights into the complexities of hormone receptor signaling at the molecular level; in vivo additional layers of complexity add on to this. The combined use of mouse genetics and tissue recombination techniques has made it possible to disentangle hormone action in vivo and revealed that estrogens, progesterone, and prolactin orchestrate distinct developmental stages of mammary gland development. The 2 ovarian steroids that fluctuate during menstrual cycles act on a subset of mammary epithelial cells, the hormone-receptor-positive sensor cells, which translate and amplify the incoming systemic signals into local, paracrine stimuli. Progesterone has emerged as a major regulator of cell proliferation and stem cell activation in the adult mammary gland. Two progesterone receptor targets, receptor activator of NfκB ligand and Wnt4, serve as downstream paracrine mediators of progesterone receptor-induced cell proliferation and stem cell activation, respectively. Some of the findings in the mouse have been validated in human ex vivo models and by next-generation whole-transcriptome sequencing on healthy donors staged for their menstrual cycles. The implications of these insights into the basic control mechanisms of mammary gland development for breast carcinogenesis and the possible role of endocrine disruptors, in particular bisphenol A in this context, will be discussed below.
Figures

References
-
- Howlader NNA, Krapcho M, Garshell J, et al. SEER Cancer Statistics Review, 1975–2011. 2014. http://seer.cancer.gov/archive/csr/1975_2011/ Accessed July 29, 2015.
-
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. Invasive breast carcinoma. In: Tavassoli F, Devilee P, eds. WHO Classification of Tumors of the Breast. vol 5 Lyon, France: IARC Press; 2012:13–59.
-
- Sonnenblick A, Fumagalli D, Sotiriou C, Piccart M. Is the differentiation into molecular subtypes of breast cancer important for staging, local and systemic therapy, and follow up? Cancer Treat Rev. 2014;40(9):1089–1095. - PubMed
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

