Intrauterine Device Placement During Cesarean Delivery and Continued Use 6 Months Postpartum: A Randomized Controlled Trial
- PMID: 26241250
- PMCID: PMC5354629
- DOI: 10.1097/AOG.0000000000000882
Intrauterine Device Placement During Cesarean Delivery and Continued Use 6 Months Postpartum: A Randomized Controlled Trial
Abstract
Objective: To compare intrauterine device (IUD) use at 6 months postpartum among women who underwent intracesarean delivery (during cesarean delivery) IUD placement compared with women who planned for interval IUD placement 6 or more weeks postpartum.
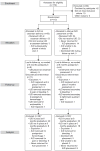
Methods: In this nonblinded randomized trial, women who were undergoing a cesarean delivery and desired an IUD were randomized to intracesarean delivery or interval IUD placement. The primary outcome was IUD use at 6 months postpartum. A sample size of 112 (56 in each group) was planned to detect a 15% difference in IUD use at 6 months postpartum between groups.
Results: From March 2012 to June 2014, 172 women were screened and 112 women were randomized into the trial. Baseline characteristics were similar between groups. Data regarding IUD use at 6 months postpartum were available for 98 women, 48 and 50 women in the intracesarean delivery and interval groups, respectively. A larger proportion of the women in the intracesarean delivery group were using an IUD at 6 months postpartum (40/48 [83%]) compared with those in the interval group (32/50 [64%], relative risk 1.3, 95% confidence interval 1.02-1.66). Among the 56 women randomized to interval IUD insertion, 22 (39%) of them never received an IUD; 14 (25%) never returned for IUD placement, five (9%) women declined an IUD, and three (5%) had a failed IUD placement.
Conclusion: Intrauterine device placement at the time of cesarean delivery leads to a higher proportion of IUD use at 6 months postpartum when compared with interval IUD placement.
Level of evidence: I.
Trial registration: ClinicalTrials.gov NCT01539759.
Figures
Comment in
-
Intrauterine Device Insertion During Cesarean Delivery: The Rising Tide of the Postdelivery Intrauterine Device.Obstet Gynecol. 2015 Jul;126(1):1-2. doi: 10.1097/AOG.0000000000000944. Obstet Gynecol. 2015. PMID: 26241248 No abstract available.
Similar articles
-
Placement of Levonorgestrel Intrauterine Device at the Time of Cesarean Delivery and the Effect on Breastfeeding Duration.Breastfeed Med. 2018 Dec;13(10):674-679. doi: 10.1089/bfm.2018.0060. Epub 2018 Oct 30. Breastfeed Med. 2018. PMID: 30376369 Free PMC article. Clinical Trial.
-
Early vs Interval Postpartum Intrauterine Device Placement: A Randomized Clinical Trial.JAMA. 2023 Mar 21;329(11):910-917. doi: 10.1001/jama.2023.1936. JAMA. 2023. PMID: 36943214 Free PMC article. Clinical Trial.
-
Intrauterine device placement at 3 versus 6 weeks postpartum: a randomized trial.Contraception. 2016 Apr;93(4):356-363. doi: 10.1016/j.contraception.2015.12.006. Epub 2015 Dec 11. Contraception. 2016. PMID: 26686914 Clinical Trial.
-
Expulsion of intrauterine devices after postpartum placement by timing of placement, delivery type, and intrauterine device type: a systematic review and meta-analysis.Am J Obstet Gynecol. 2020 Aug;223(2):177-188. doi: 10.1016/j.ajog.2020.02.045. Epub 2020 Mar 3. Am J Obstet Gynecol. 2020. PMID: 32142826 Free PMC article.
-
Society of Family Planning Guidelines: Postplacental insertion of intrauterine devices.Contraception. 2018 Jan;97(1):2-13. doi: 10.1016/j.contraception.2017.09.014. Epub 2017 Oct 5. Contraception. 2018. PMID: 28987293 Review.
Cited by
-
Uptake of postplacental intrauterine device placement at cesarean delivery.AJOG Glob Rep. 2023 Jan 4;3(1):100157. doi: 10.1016/j.xagr.2022.100157. eCollection 2023 Feb. AJOG Glob Rep. 2023. PMID: 36748028 Free PMC article.
-
A Decision Analysis Model of 1-Year Effectiveness of Intended Postplacental Compared With Intended Delayed Postpartum Intrauterine Device Insertion.Obstet Gynecol. 2018 Nov;132(5):1211-1221. doi: 10.1097/AOG.0000000000002926. Obstet Gynecol. 2018. PMID: 30303909 Free PMC article.
-
One-year continuation of copper or levonorgestrel intrauterine devices initiated at the time of emergency contraception.Contraception. 2017 Aug;96(2):99-105. doi: 10.1016/j.contraception.2017.05.012. Epub 2017 Jun 5. Contraception. 2017. PMID: 28596121 Free PMC article.
-
Insertion of intrauterine devices after cesarean section: a systematic review update.Int J Womens Health. 2017 Apr 18;9:205-212. doi: 10.2147/IJWH.S132391. eCollection 2017. Int J Womens Health. 2017. PMID: 28458581 Free PMC article. Review.
-
Ultrasound assessment of postplacental copper intrauterine device position 6 months after placement during cesarean delivery.Contracept X. 2020 Oct 9;2:100040. doi: 10.1016/j.conx.2020.100040. eCollection 2020. Contracept X. 2020. PMID: 33196037 Free PMC article.
References
-
- Tocce KM, Sheeder JL, Teal SB. Rapid repeat pregnancy in adolescents: do immediate postpartum contraceptive implants make a difference? American journal of obstetrics and gynecology. 2012 Jun;206(6):481 e481–487. - PubMed
-
- Speroff L, Mishell DR., Jr The postpartum visit: it's time for a change in order to optimally initiate contraception. Contraception. 2008 Aug;78(2):90–98. - PubMed
-
- Ogburn JA, Espey E, Stonehocker J. Barriers to intrauterine device insertion in postpartum women. Contraception. 2005 Dec;72(6):426–429. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials