Abnormal subcellular localization of GABAA receptor subunits in schizophrenia brain
- PMID: 26241350
- PMCID: PMC4564557
- DOI: 10.1038/tp.2015.102
Abnormal subcellular localization of GABAA receptor subunits in schizophrenia brain
Abstract
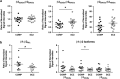
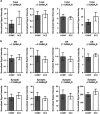
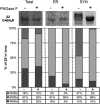
Inhibitory neurotransmission is primarily mediated by γ-aminobutyric acid (GABA) activating synaptic GABA type A receptors (GABA(A)R). In schizophrenia, presynaptic GABAergic signaling deficits are among the most replicated findings; however, postsynaptic GABAergic deficits are less well characterized. Our lab has previously demonstrated that although there is no difference in total protein expression of the α1-6, β1-3 or γ2 GABA(A)R subunits in the superior temporal gyrus (STG) in schizophrenia, the α1, β1 and β2 GABA(A)R subunits are abnormally N-glycosylated. N-glycosylation is a posttranslational modification that has important functional roles in protein folding, multimer assembly and forward trafficking. To investigate the impact that altered N-glycosylation has on the assembly and trafficking of GABA(A)Rs in schizophrenia, this study used western blot analysis to measure the expression of α1, α2, β1, β2 and γ2 GABA(A)R subunits in subcellular fractions enriched for endoplasmic reticulum (ER) and synapses (SYN) from STG of schizophrenia (N = 16) and comparison (N = 14) subjects and found evidence of abnormal localization of the β1 and β2 GABA(A)R subunits and subunit isoforms in schizophrenia. The β2 subunit is expressed as three isoforms at 52 kDa (β2(52 kDa)), 50 kDa (β2(50 kDa)) and 48 kDa (β2(48 kDa)). In the ER, we found increased total β2 GABA(A)R subunit (β2(ALL)) expression driven by increased β2(50 kDa), a decreased ratio of β(248 kDa):β2(ALL) and an increased ratio of β2(50 kDa):β2(48 kDa). Decreased ratios of β1:β2(ALL) and β1:β2(50 kDa) in both the ER and SYN fractions and an increased ratio of β2(52 kDa):β(248 kDa) at the synapse were also identified in schizophrenia. Taken together, these findings provide evidence that alterations of N-glycosylation may contribute to GABAergic signaling deficits in schizophrenia by disrupting the assembly and trafficking of GABA(A)Rs.
Figures


Similar articles
-
N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia.Neuropsychopharmacology. 2014 Feb;39(3):528-37. doi: 10.1038/npp.2013.190. Epub 2013 Aug 6. Neuropsychopharmacology. 2014. PMID: 23917429 Free PMC article.
-
GABAA and GABAB receptor dysregulation in superior frontal cortex of subjects with schizophrenia and bipolar disorder.Synapse. 2017 Jul;71(7). doi: 10.1002/syn.21973. Epub 2017 Apr 5. Synapse. 2017. PMID: 28316115
-
Developmental expression and distribution of GABA(A) receptor α1-, α3- and β2-subunits in pig brain.Dev Neurosci. 2011;33(2):99-109. doi: 10.1159/000326630. Epub 2011 May 25. Dev Neurosci. 2011. PMID: 21613774
-
[Schizophrenia and cortical GABA neurotransmission].Seishin Shinkeigaku Zasshi. 2010;112(5):439-52. Seishin Shinkeigaku Zasshi. 2010. PMID: 20560363 Review. Japanese.
-
The diversity of GABA(A) receptor subunit distribution in the normal and Huntington's disease human brain.Adv Pharmacol. 2015;73:223-64. doi: 10.1016/bs.apha.2014.11.010. Epub 2015 Jan 17. Adv Pharmacol. 2015. PMID: 25637443 Review.
Cited by
-
The dynamic brain N-glycome.Glycoconj J. 2022 Jun;39(3):443-471. doi: 10.1007/s10719-022-10055-x. Epub 2022 Mar 25. Glycoconj J. 2022. PMID: 35334027 Review.
-
Abnormal N-acetylglucosaminyltransferase expression in prefrontal cortex in schizophrenia.Schizophr Res. 2015 Aug;166(1-3):219-24. doi: 10.1016/j.schres.2015.06.002. Epub 2015 Jun 20. Schizophr Res. 2015. PMID: 26104473 Free PMC article.
-
Mechanism of BDNF Modulation in GABAergic Synaptic Transmission in Healthy and Disease Brains.Front Cell Neurosci. 2018 Aug 28;12:273. doi: 10.3389/fncel.2018.00273. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30210299 Free PMC article.
-
Abnormal ER quality control of neural GPI-anchored proteins via dysfunction in ER export processing in the frontal cortex of elderly subjects with schizophrenia.Transl Psychiatry. 2019 Jan 16;9(1):6. doi: 10.1038/s41398-018-0359-4. Transl Psychiatry. 2019. PMID: 30664618 Free PMC article.
-
GABARAPs dysfunction by autophagy deficiency in adolescent brain impairs GABAA receptor trafficking and social behavior.Sci Adv. 2019 Apr 10;5(4):eaau8237. doi: 10.1126/sciadv.aau8237. eCollection 2019 Apr. Sci Adv. 2019. PMID: 30989111 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

