Pooled Analysis of Six Pharmacologic and Nonpharmacologic Interventions for Vasomotor Symptoms
- PMID: 26241433
- PMCID: PMC4526122
- DOI: 10.1097/AOG.0000000000000927
Pooled Analysis of Six Pharmacologic and Nonpharmacologic Interventions for Vasomotor Symptoms
Abstract
Objective: To describe the effects of six interventions for menopausal vasomotor symptoms relative to control in a pooled analysis, facilitating translation of the results for clinicians and symptomatic women. The Menopause Strategies: Finding Lasting Answers for Symptoms and Health network tested these interventions in three randomized clinical trials.
Methods: An analysis of pooled individual-level data from three randomized clinical trials is presented. Participants were 899 perimenopausal and postmenopausal women with at least 14 bothersome vasomotor symptoms per week. Interventions included 10-20 mg escitalopram per day, nonaerobic yoga, aerobic exercise, 1.8 g per day omega-3 fatty acid supplementation, 0.5 mg low-dose oral 17-beta-estradiol (E2) per day, and 75 mg low-dose venlafaxine XR per day. The main outcome measures were changes from baseline in mean daily vasomotor symptom frequency and bother during 8-12 weeks of treatment. Linear regression models estimated differences in outcomes between each intervention and corresponding control group adjusted for baseline characteristics. Models included trial-specific intercepts, effects of the baseline outcome measure, and time.
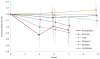
Results: The 8-week reduction in vasomotor symptom frequency from baseline relative to placebo was similar for escitalopram at -1.4 per day (95% confidence interval [CI] -2.7 to -0.2), low-dose E2 at -2.4 (95% CI -3.4 to -1.3), and venlafaxine at -1.8 (95% CI -2.8 to -0.8); vasomotor symptom bother reduction was minimal and did not vary across these three pharmacologic interventions (mean -0.2 to -0.3 relative to placebo). No effects on vasomotor symptom frequency or bother were seen with aerobic exercise, yoga, or omega-3 supplements.
Conclusion: These analyses suggest that escitalopram, low-dose E2, and venlafaxine provide comparable, modest reductions in vasomotor symptom frequency and bother among women with moderate hot flushes.
Clinical trial registration: ClinicalTrials.gov, www.clinicaltrials.gov, NCT00894543 (MsFLASH 01), NCT01178892 (MsFLASH 02), and NCT01418209 (MsFLASH 03).
Figures
References
-
- NIH State-of-the-Science Panel, National Institutes of Health State-of-the-Science Conference Statement. Management of Menopause-Related Symptoms. Ann Intern Med. 2005;142:1003–13. - PubMed
-
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. - PubMed
-
- Grady D, Cohen B, Tice J, Kristof M, Olyaie A, Sawaya GF. Ineffectiveness of sertraline for treatment of menopausal hot flushes: a randomized controlled trial. Obstet Gynecol. 2007;109:823–30. - PubMed
-
- Speroff L, Gass M, Constantine G, Olivier S. Efficacy and tolerability of desvenlafaxine succinate treatment for menopausal vasomotor symptoms. Obstet Gynecol. 2008;111:77–87. - PubMed
-
- Butt DA, Lock M, Lewis JE, Ross S, Moineddin R. Gabapentin for the treatment of menopausal hot flashes: a randomized controlled trial. Menopause. 2008;15:310–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U01 DK60401/DK/NIDDK NIH HHS/United States
- U01 DK58234/DK/NIDDK NIH HHS/United States
- U01 AG032682/AG/NIA NIH HHS/United States
- U01 DK60379/DK/NIDDK NIH HHS/United States
- U01 AG032669/AG/NIA NIH HHS/United States
- UL1 TR001108/TR/NCATS NIH HHS/United States
- R01 AG048209/AG/NIA NIH HHS/United States
- U01 DK60395/DK/NIDDK NIH HHS/United States
- U01 DK60397/DK/NIDDK NIH HHS/United States
- U01 DK58231/DK/NIDDK NIH HHS/United States
- U01 DK60393/DK/NIDDK NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- U01 AG032659/AG/NIA NIH HHS/United States
- U01 DK60380/DK/NIDDK NIH HHS/United States
- U01 AG032699/AG/NIA NIH HHS/United States
- U01 DK58225/DK/NIDDK NIH HHS/United States
- U01 AG032700/AG/NIA NIH HHS/United States
- U01 DK58229/DK/NIDDK NIH HHS/United States