Connective-Tissue Growth Factor (CTGF/CCN2) Induces Astrogenesis and Fibronectin Expression of Embryonic Neural Cells In Vitro
- PMID: 26241738
- PMCID: PMC4524627
- DOI: 10.1371/journal.pone.0133689
Connective-Tissue Growth Factor (CTGF/CCN2) Induces Astrogenesis and Fibronectin Expression of Embryonic Neural Cells In Vitro
Abstract
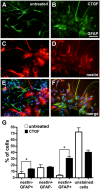
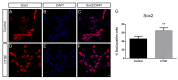
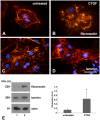
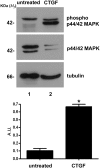
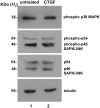
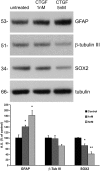
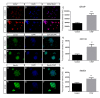
Connective-tissue growth factor (CTGF) is a modular secreted protein implicated in multiple cellular events such as chondrogenesis, skeletogenesis, angiogenesis and wound healing. CTGF contains four different structural modules. This modular organization is characteristic of members of the CCN family. The acronym was derived from the first three members discovered, cysteine-rich 61 (CYR61), CTGF and nephroblastoma overexpressed (NOV). CTGF is implicated as a mediator of important cell processes such as adhesion, migration, proliferation and differentiation. Extensive data have shown that CTGF interacts particularly with the TGFβ, WNT and MAPK signaling pathways. The capacity of CTGF to interact with different growth factors lends it an important role during early and late development, especially in the anterior region of the embryo. ctgf knockout mice have several cranio-facial defects, and the skeletal system is also greatly affected due to an impairment of the vascular-system development during chondrogenesis. This study, for the first time, indicated that CTGF is a potent inductor of gliogenesis during development. Our results showed that in vitro addition of recombinant CTGF protein to an embryonic mouse neural precursor cell culture increased the number of GFAP- and GFAP/Nestin-positive cells. Surprisingly, CTGF also increased the number of Sox2-positive cells. Moreover, this induction seemed not to involve cell proliferation. In addition, exogenous CTGF activated p44/42 but not p38 or JNK MAPK signaling, and increased the expression and deposition of the fibronectin extracellular matrix protein. Finally, CTGF was also able to induce GFAP as well as Nestin expression in a human malignant glioma stem cell line, suggesting a possible role in the differentiation process of gliomas. These results implicate ctgf as a key gene for astrogenesis during development, and suggest that its mechanism may involve activation of p44/42 MAPK signaling. Additionally, CTGF-induced differentiation of glioblastoma stem cells into a less-tumorigenic state could increase the chances of successful intervention, since differentiated cells are more vulnerable to cancer treatments.
Conflict of interest statement
Figures
Similar articles
-
Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61).Angiogenesis. 2002;5(3):153-65. doi: 10.1023/a:1023823803510. Angiogenesis. 2002. PMID: 12831056
-
Connective tissue growth factor (CTGF/CCN2) is negatively regulated during neuron-glioblastoma interaction.PLoS One. 2013;8(1):e55605. doi: 10.1371/journal.pone.0055605. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383241 Free PMC article.
-
PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo.Genes Dev. 2001 Aug 1;15(15):1913-25. doi: 10.1101/gad.903001. Genes Dev. 2001. PMID: 11485986 Free PMC article.
-
Regulation of hepatic stellate cells by connective tissue growth factor.Front Biosci (Landmark Ed). 2012 Jun 1;17(7):2495-507. doi: 10.2741/4067. Front Biosci (Landmark Ed). 2012. PMID: 22652794 Review.
-
The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis.Crit Rev Eukaryot Gene Expr. 2011;21(1):43-69. doi: 10.1615/critreveukargeneexpr.v21.i1.40. Crit Rev Eukaryot Gene Expr. 2011. PMID: 21967332 Free PMC article. Review.
Cited by
-
Development of a Single-Neurosphere Culture to Assess Radiation Toxicity and Pre-Clinical Cancer Combination Therapy Safety.Cancers (Basel). 2023 Oct 10;15(20):4916. doi: 10.3390/cancers15204916. Cancers (Basel). 2023. PMID: 37894283 Free PMC article.
-
Influence of microcurrent on the modulation of remodelling genes in a wound healing assay.Mol Biol Rep. 2021 Feb;48(2):1233-1241. doi: 10.1007/s11033-021-06135-0. Epub 2021 Jan 21. Mol Biol Rep. 2021. PMID: 33475929
-
Protective Effects of Platelet-rich plasma for in vitro Fertilization of Rats with Ovarian Failure Induced by Cyclophosphamide.Rev Bras Ginecol Obstet. 2022 Feb;44(2):161-168. doi: 10.1055/s-0041-1741451. Epub 2022 Feb 25. Rev Bras Ginecol Obstet. 2022. PMID: 35213914 Free PMC article.
-
Connective tissue growth factor: Role in trabecular meshwork remodeling and intraocular pressure lowering.Exp Biol Med (Maywood). 2023 Aug;248(16):1425-1436. doi: 10.1177/15353702231199466. Epub 2023 Oct 24. Exp Biol Med (Maywood). 2023. PMID: 37873757 Free PMC article. Review.
-
Molecular laterality encodes stress susceptibility in the medial prefrontal cortex.Mol Brain. 2021 Jun 14;14(1):92. doi: 10.1186/s13041-021-00802-w. Mol Brain. 2021. PMID: 34127022 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

