Neuroimmune and Neuropathic Responses of Spinal Cord and Dorsal Root Ganglia in Middle Age
- PMID: 26241743
- PMCID: PMC4524632
- DOI: 10.1371/journal.pone.0134394
Neuroimmune and Neuropathic Responses of Spinal Cord and Dorsal Root Ganglia in Middle Age
Abstract
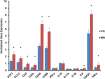
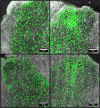
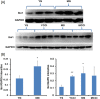
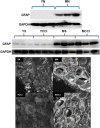
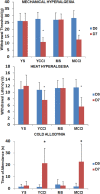
Prior studies of aging and neuropathic injury have focused on senescent animals compared to young adults, while changes in middle age, particularly in the dorsal root ganglia (DRG), have remained largely unexplored. 14 neuroimmune mRNA markers, previously associated with peripheral nerve injury, were measured in multiplex assays of lumbar spinal cord (LSC), and DRG from young and middle-aged (3, 17 month) naïve rats, or from rats subjected to chronic constriction injury (CCI) of the sciatic nerve (after 7 days), or from aged-matched sham controls. Results showed that CD2, CD3e, CD68, CD45, TNF-α, IL6, CCL2, ATF3 and TGFβ1 mRNA levels were substantially elevated in LSC from naïve middle-aged animals compared to young adults. Similarly, LSC samples from older sham animals showed increased levels of T-cell and microglial/macrophage markers. CCI induced further increases in CCL2, and IL6, and elevated ATF3 mRNA levels in LSC of young and middle-aged adults. Immunofluorescence images of dorsal horn microglia from middle-aged naïve or sham rats were typically hypertrophic with mostly thickened, de-ramified processes, similar to microglia following CCI. Unlike the spinal cord, marker expression profiles in naïve DRG were unchanged across age (except increased ATF3); whereas, levels of GFAP protein, localized to satellite glia, were highly elevated in middle age, but independent of nerve injury. Most neuroimmune markers were elevated in DRG following CCI in young adults, yet middle-aged animals showed little response to injury. No age-related changes in nociception (heat, cold, mechanical) were observed in naïve adults, or at days 3 or 7 post-CCI. The patterns of marker expression and microglial morphologies in healthy middle age are consistent with development of a para-inflammatory state involving microglial activation and T-cell marker elevation in the dorsal horn, and neuronal stress and satellite cell activation in the DRG. These changes, however, did not affect the establishment of neuropathic pain.
Conflict of interest statement
Figures




References
-
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mechanisms of ageing and development. 2007;128(1):92–105. Epub 2006/11/23. 10.1016/j.mad.2006.11.016 . - DOI - PubMed
-
- Franceschi C, Valensin S, Lescai F, Olivieri F, Licastro F, Grimaldi LM, et al. Neuroinflammation and the genetics of Alzheimer's disease: the search for a pro-inflammatory phenotype. Aging (Milano). 2001;13(3):163–70. Epub 2001/07/10. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

