Hematopoietic progenitors are required for proper development of coronary vasculature
- PMID: 26241844
- PMCID: PMC4558312
- DOI: 10.1016/j.yjmcc.2015.07.021
Hematopoietic progenitors are required for proper development of coronary vasculature
Abstract
Rationale: During embryogenesis, hematopoietic cells appear in the myocardium prior to the initiation of coronary formation. However, their role is unknown.
Objective: Here we investigate whether pre-existing hematopoietic cells are required for the formation of coronary vasculature.
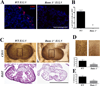
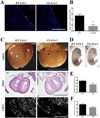
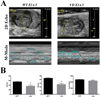
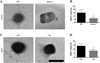
Methods and results: As a model of for hematopoietic cell deficient animals, we used Runx1 knockout embryos and Vav1-cre; R26-DTA embryos, latter of which genetically ablates 2/3 of CD45(+) hematopoietic cells. Both Runx1 knockout embryos and Vav1-cre; R26-DTA embryos revealed disorganized, hypoplastic microvasculature of coronary vessels on section and whole-mount stainings. Furthermore, coronary explant experiments showed that the mouse heart explants from Runx1 and Vav1-cre; R26-DTA embryos exhibited impaired coronary formation ex vivo. Interestingly, in both models it appears that epicardial to mesenchymal transition is adversely affected in the absence of hematopoietic progenitors.
Conclusion: Hematopoietic cells are not merely passively transported via coronary vessel, but substantially involved in the induction of the coronary growth. Our findings suggest a novel mechanism of coronary growth.
Keywords: Cardiac development; Coronary formation; EMT; Epicardium; Hematopoietic progenitors.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosures and conflicts of interest: None
Figures
References
-
- Mathers C, Fat DM, Boerma JT World Health Organization. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization; 2008.
-
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. - PubMed
-
- Aikawa E, Kawano J. Formation of coronary arteries sprouting from the primitive aortic sinus wall of the chick embryo. Experientia. 1982;38:816–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

