Markers of β-Cell Failure Predict Poor Glycemic Response to GLP-1 Receptor Agonist Therapy in Type 2 Diabetes
- PMID: 26242184
- PMCID: PMC4894547
- DOI: 10.2337/dc15-0258
Markers of β-Cell Failure Predict Poor Glycemic Response to GLP-1 Receptor Agonist Therapy in Type 2 Diabetes
Abstract
Objective: To assess whether clinical characteristics and simple biomarkers of β-cell failure are associated with individual variation in glycemic response to GLP-1 receptor agonist (GLP-1RA) therapy in patients with type 2 diabetes.
Research design and methods: We prospectively studied 620 participants with type 2 diabetes and HbA1c ≥58 mmol/mol (7.5%) commencing GLP-1RA therapy as part of their usual diabetes care and assessed response to therapy over 6 months. We assessed the association between baseline clinical measurements associated with β-cell failure and glycemic response (primary outcome HbA1c change 0-6 months) with change in weight (0-6 months) as a secondary outcome using linear regression and ANOVA with adjustment for baseline HbA1c and cotreatment change.
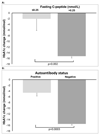
Results: Reduced glycemic response to GLP-1RAs was associated with longer duration of diabetes, insulin cotreatment, lower fasting C-peptide, lower postmeal urine C-peptide-to-creatinine ratio, and positive GAD or IA2 islet autoantibodies (P ≤ 0.01 for all). Participants with positive autoantibodies or severe insulin deficiency (fasting C-peptide ≤0.25 nmol/L) had markedly reduced glycemic response to GLP-1RA therapy (autoantibodies, mean HbA1c change -5.2 vs. -15.2 mmol/mol [-0.5 vs. -1.4%], P = 0.005; C-peptide <0.25 nmol/L, mean change -2.1 vs. -15.3 mmol/mol [-0.2 vs. -1.4%], P = 0.002). These markers were predominantly present in insulin-treated participants and were not associated with weight change.
Conclusions: Clinical markers of low β-cell function are associated with reduced glycemic response to GLP-1RA therapy. C-peptide and islet autoantibodies represent potential biomarkers for the stratification of GLP-1RA therapy in insulin-treated diabetes.
Trial registration: ClinicalTrials.gov NCT01503112.
© 2016 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures


References
-
- National Institute of Clinical Excellence (UK) Guideline CG87 - Type 2 diabetes: the management of type 2 diabetes. 2009
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. - PubMed
-
- Thong KY, Jose B, Sukumar N, Cull ML, Mills AP, Sathyapalan T, Shafiq W, Rigby AS, Walton C, Ryder RE. Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Diabetologists (ABCD) nationwide exenatide audit. Diabetes, Obesity & Metabolism. 2011;13:703–10. - PubMed
-
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. - PubMed
-
- Hirsch IB, Molitch ME. Clinical decisions. Glycemic management in a patient with type 2 diabetes. The New England Journal of Medicine. 2013;369:1370–1372. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

