Essential metals at the host-pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens
- PMID: 26242402
- PMCID: PMC4629794
- DOI: 10.1093/femsyr/fov071
Essential metals at the host-pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens
Abstract
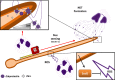
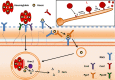
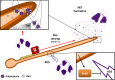
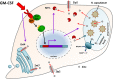
The ability of pathogenic microorganisms to assimilate sufficient nutrients for growth within their hosts is a fundamental requirement for pathogenicity. However, certain trace nutrients, including iron, zinc and manganese, are actively withheld from invading pathogens in a process called nutritional immunity. Therefore, successful pathogenic species must have evolved specialized mechanisms in order to adapt to the nutritionally restrictive environment of the host and cause disease. In this review, we discuss recent advances which have been made in our understanding of fungal iron and zinc acquisition strategies and nutritional immunity against fungal infections, and explore the mechanisms of micronutrient uptake by human pathogenic fungi.
Keywords: fungal pathogenicity; host–pathogen interactions; iron; zinc.
© FEMS 2015.
Figures


References
-
- Almeida RS, Wilson D, Hube B. Candida albicans iron acquisition within the host. FEMS Yeast Res. 2009;9:1000–12. - PubMed
-
- Amich J, Vicentefranqueira R, Mellado E, et al. The ZrfC alkaline zinc transporter is required for Aspergillus fumigatus virulence and its growth in the presence of the Zn/Mn-chelating protein calprotectin. Cell Microbiol. 2014;16:548–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

