Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation
- PMID: 26242575
- PMCID: PMC4824045
- DOI: 10.1038/ncomms8965
Pannexin 1 channels regulate leukocyte emigration through the venous endothelium during acute inflammation
Abstract
Inflammatory cell recruitment to local sites of tissue injury and/or infection is controlled by a plethora of signalling processes influencing cell-to-cell interactions between the vascular endothelial cells (ECs) in post-capillary venules and circulating leukocytes. Recently, ATP-sensitive P2Y purinergic receptors have emerged as downstream regulators of EC activation in vascular inflammation. However, the mechanism(s) regulating cellular ATP release in this response remains elusive. Here we report that the ATP-release channel Pannexin1 (Panx1) opens downstream of EC activation by TNF-α. This process involves activation of type-1 TNF receptors, recruitment of Src family kinases (SFK) and SFK-dependent phosphorylation of Panx1. Using an inducible, EC-specific Panx1 knockout mouse line, we report a previously unidentified role for Panx1 channels in promoting leukocyte adhesion and emigration through the venous wall during acute systemic inflammation, placing Panx1 channels at the centre of cytokine crosstalk with purinergic signalling in the endothelium.
Figures


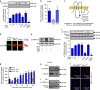
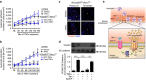
References
-
- Ley K., Laudanna C., Cybulsky M. I. & Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 (2007). - PubMed
-
- Ralevic V. & Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492 (1998). - PubMed
-
- Zerr M. et al. Major contribution of the P2Y(1)receptor in purinergic regulation of TNFalpha-induced vascular inflammation. Circulation 123, 2404–2413 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

