Structural Insight into Specificity of Interactions between Nonconventional Three-finger Weak Toxin from Naja kaouthia (WTX) and Muscarinic Acetylcholine Receptors
- PMID: 26242733
- PMCID: PMC4583006
- DOI: 10.1074/jbc.M115.656595
Structural Insight into Specificity of Interactions between Nonconventional Three-finger Weak Toxin from Naja kaouthia (WTX) and Muscarinic Acetylcholine Receptors
Abstract
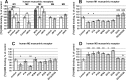
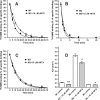
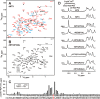
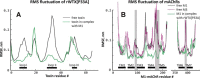
Weak toxin from Naja kaouthia (WTX) belongs to the group of nonconventional "three-finger" snake neurotoxins. It irreversibly inhibits nicotinic acetylcholine receptors and allosterically interacts with muscarinic acetylcholine receptors (mAChRs). Using site-directed mutagenesis, NMR spectroscopy, and computer modeling, we investigated the recombinant mutant WTX analogue (rWTX) which, compared with the native toxin, has an additional N-terminal methionine residue. In comparison with the wild-type toxin, rWTX demonstrated an altered pharmacological profile, decreased binding of orthosteric antagonist N-methylscopolamine to human M1- and M2-mAChRs, and increased antagonist binding to M3-mAChR. Positively charged arginine residues located in the flexible loop II were found to be crucial for rWTX interactions with all types of mAChR. Computer modeling suggested that the rWTX loop II protrudes to the M1-mAChR allosteric ligand-binding site blocking the entrance to the orthosteric site. In contrast, toxin interacts with M3-mAChR by loop II without penetration into the allosteric site. Data obtained provide new structural insight into the target-specific allosteric regulation of mAChRs by "three-finger" snake neurotoxins.
Keywords: G protein-coupled receptor (GPCR); computer modeling; nuclear magnetic resonance (NMR); protein dynamic; recombinant protein expression; site-directed mutagenesis; snake neurotoxin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures




References
-
- Tsetlin V. I., Hucho F. (2004) Snake and snail toxins acting on nicotinic acetylcholine receptors: fundamental aspects and medical applications. FEBS Lett. 557, 9–13 - PubMed
-
- Kini R. M., Doley R. (2010) Structure, function and evolution of three-finger toxins: mini proteins with multiple targets. Toxicon 56, 855–867 - PubMed
-
- Wess J., Eglen R. M., Gautam D. (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat. Rev. Drug Discov. 6, 721–733 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

