Heat stress increases insulin sensitivity in pigs
- PMID: 26243213
- PMCID: PMC4562564
- DOI: 10.14814/phy2.12478
Heat stress increases insulin sensitivity in pigs
Abstract
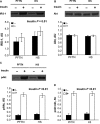
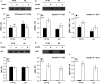
Proper insulin homeostasis appears critical for adapting to and surviving a heat load. Further, heat stress (HS) induces phenotypic changes in livestock that suggest an increase in insulin action. The current study objective was to evaluate the effects of HS on whole-body insulin sensitivity. Female pigs (57 ± 4 kg body weight) were subjected to two experimental periods. During period 1, all pigs remained in thermoneutral conditions (TN; 21°C) and were fed ad libitum. During period 2, pigs were exposed to: (i) constant HS conditions (32°C) and fed ad libitum (n = 6), or (ii) TN conditions and pair-fed (PFTN; n = 6) to eliminate the confounding effects of dissimilar feed intake. A hyperinsulinemic euglycemic clamp (HEC) was conducted on d3 of both periods; and skeletal muscle and adipose tissue biopsies were collected prior to and after an insulin tolerance test (ITT) on d5 of period 2. During the HEC, insulin infusion increased circulating insulin and decreased plasma C-peptide and nonesterified fatty acids, similarly between treatments. From period 1 to 2, the rate of glucose infusion in response to the HEC remained similar in HS pigs while it decreased (36%) in PFTN controls. Prior to the ITT, HS increased (41%) skeletal muscle insulin receptor substrate-1 protein abundance, but did not affect protein kinase B or their phosphorylated forms. In adipose tissue, HS did not alter any of the basal or stimulated measured insulin signaling markers. In summary, HS increases whole-body insulin-stimulated glucose uptake.
Keywords: Heat Stress; insulin sensitivity; metabolism; pig.
© 2015 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

References
-
- Achmadi J, Yanagisawa T, Sano H. Terashima Y. Pancreatic insulin secretory response and insulin action in heat-exposed sheep given a concentrate or roughage diet. Domest. Anim. Endocrinol. 1993;10:279–287. - PubMed
-
- Ahmed K, Tunaru S, Tang C, Muller M, Gille A, Sassmann A, et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 2010;11:311–319. - PubMed
-
- Alamer M. The role of prolactin in thermoregulation and water balance during heat stress in domestic ruminants. Asian J. Anim. Vet. Adv. 2011;6:1153–1169.
-
- Angus DJ, Febbraio MA, Lasini D. Hargreaves M. Effect of carbohydrate ingestion on glucose kinetics during exercise in the heat. J. Appl. Physiol. 1985;90:2001. - PubMed
-
- Bauman DE. Currie WB. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980;63:1514–1529. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

