Combined Approach to Lysis Utilizing Eptifibatide and Recombinant Tissue-Type Plasminogen Activator in Acute Ischemic Stroke-Full Dose Regimen Stroke Trial
- PMID: 26243231
- PMCID: PMC4550507
- DOI: 10.1161/STROKEAHA.115.010260
Combined Approach to Lysis Utilizing Eptifibatide and Recombinant Tissue-Type Plasminogen Activator in Acute Ischemic Stroke-Full Dose Regimen Stroke Trial
Abstract
Background and purpose: The Combined Approach to Lysis Utilizing Eptifibatide and Recombinant Tissue-Type Plasminogen Activator (r-tPA; CLEAR) in Acute Ischemic Stroke (AIS) and CLEAR-Enhanced Regimen (CLEAR-ER) trials demonstrated safety of reduced dose r-tPA plus the glycoprotein 2b/3a inhibitor, eptifibatide, in AIS compared with r-tPA alone. The objective of the CLEAR-Full Dose Regimen (CLEAR-FDR) trial was to estimate the rate of symptomatic intracerebral hemorrhage (sICH) in AIS patients treated with the combination of full-dose r-tPA plus eptifibatide.
Methods: CLEAR-FDR was a single-arm, prospective, open-label, multisite study. Patients aged 18 to 85 years treated with 0.9 mg/kg IV r-tPA within 3 hours of symptom onset were enrolled. After obtaining consent, eptifibatide (135 μg/kg bolus and 2-hour infusion at 0.75 μg/kg per minute) was administered. The primary end point was the proportion of patients who experienced sICH within 36 hours. An independent clinical monitor adjudicated if an sICH had occurred and an independent neuroradiologist reviewed all images. The stopping rule was 3 sICHs within the first 19 patients or 4 sICHs within 29 patients.
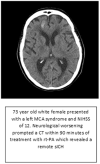
Results: From October 2013 to December 2014, 27 patients with AIS were enrolled. Median age was 73 years (range, 34-85; interquartile range, 65-80) and median National Institute of Health stroke scale score was 12 (range, 6-26; interquartile range, 9-16). One sICH (3.7%; 95% confidence interval, 0.7%-18%) was observed.
Conclusions: These results demonstrate comparable safety of full-dose r-tPA plus eptifibatide with historical rates of sICH with r-tPA alone and support proceeding with a phase 3 trial evaluating full-dose r-tPA combined with eptifibatide to improve outcomes after AIS.
Keywords: clinical trial; eptifibatide; stroke; tissue-type plasminogen activator.
© 2015 American Heart Association, Inc.
Figures
References
-
- Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke; a journal of cerebral circulation. 2007;38:967–973. - PubMed
-
- Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59:862–867. - PubMed
-
- Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. The New England journal of medicine. 2004;351:2170–2178. - PubMed
-
- Rubiera M, Alvarez-Sabin J, Ribo M, Montaner J, Santamarina E, Arenillas JF, et al. Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke; a journal of cerebral circulation. 2005;36:1452–1456. - PubMed