Translating transitions - how to decipher peripheral human B cell development
- PMID: 26243514
- PMCID: PMC4547376
- DOI: 10.7555/JBR.29.20150035
Translating transitions - how to decipher peripheral human B cell development
Abstract
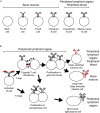
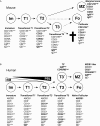
During the last two decades our understanding of human B cell differentiation has developed considerably. Our understanding of the human B cell compartment has advanced from a point where essentially all assays were based on the presence or not of class-switched antibodies to a level where a substantial diversity is appreciated among the cells involved. Several consecutive transitional stages that newly formed IgM expressing B cells go through after they leave the bone marrow, but before they are fully mature, have been described, and a significant complexity is also acknowledged within the IgM expressing and class-switched memory B cell compartments. It is possible to isolate plasma blasts in blood to follow the formation of plasma cells during immune responses, and the importance and uniqueness of the mucosal IgA system is now much more appreciated. Current data suggest the presence of at least one lineage of human innate-like B cells akin to B1 and/or marginal zone B cells in mice. In addition, regulatory B cells with the ability to produce IL-10 have been identified. Clinically, B cell depletion therapy is used for a broad range of conditions. The ability to define different human B cell subtypes using flow cytometry has therefore started to come into clinical use, but as our understanding of human B cell development further progresses, B cell subtype analysis will be of increasing importance in diagnosis, to measure the effect of immune therapy and to understand the underlying causes for diseases. In this review the diversity of human B cells will be discussed, with special focus on current data regarding their phenotypes and functions.
© 2015 the Journal of Biomedical Research. All rights reserved.
Conflict of interest statement
CLC number: R372.1, Document code: A
The authors reported no conflict of interests.
Figures

References
-
- Rosado MM, Aranburu A, Capolunghi F, et al. From the fetal liver to spleen and gut: the highway to natural antibody[J] Mucosal Immunol. 2009;2(4):351–361. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

