A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome
- PMID: 26243569
- PMCID: PMC4678594
- DOI: 10.1038/nrn3983
A genetic cause of Alzheimer disease: mechanistic insights from Down syndrome
Abstract
Down syndrome, which arises in individuals carrying an extra copy of chromosome 21, is associated with a greatly increased risk of early-onset Alzheimer disease. It is thought that this risk is conferred by the presence of three copies of the gene encoding amyloid precursor protein (APP)--an Alzheimer disease risk factor--although the possession of extra copies of other chromosome 21 genes may also play a part. Further study of the mechanisms underlying the development of Alzheimer disease in people with Down syndrome could provide insights into the mechanisms that cause dementia in the general population.
Figures

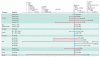
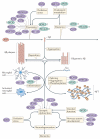
References
-
- Lejeune J, Gautier M, Turpin R. Etude des chromosomes somatiques de neuf enfants mongoliens. C. R. Hebd. Seances Acad. Sci. 1959;248:1721–1722. (in French) - PubMed
-
- de Graaf G, Buckley F, Skotko BG. Estimates of the live births, natural losses, and elective terminations with Down syndrome in the United States. Am. J. Med. Genet. A. 2015;167A:756–767. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

