Rice TUTOU1 Encodes a Suppressor of cAMP Receptor-Like Protein That Is Important for Actin Organization and Panicle Development
- PMID: 26243616
- PMCID: PMC4587440
- DOI: 10.1104/pp.15.00229
Rice TUTOU1 Encodes a Suppressor of cAMP Receptor-Like Protein That Is Important for Actin Organization and Panicle Development
Abstract
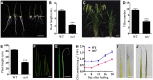
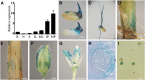
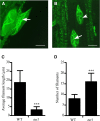
Panicle development, a key event in rice (Oryza sativa) reproduction and a critical determinant of grain yield, forms a branched structure containing multiple spikelets. Genetic and environmental factors can perturb panicle development, causing panicles to degenerate and producing characteristic whitish, small spikelets with severely reduced fertility and yield; however, little is known about the molecular basis of the formation of degenerating panicles in rice. Here, we report the identification and characterization of the rice panicle degenerative mutant tutou1 (tut1), which shows severe defects in panicle development. The tut1 also shows a pleiotropic phenotype, characterized by short roots, reduced plant height, and abnormal development of anthers and pollen grains. Molecular genetic studies revealed that TUT1 encodes a suppressor of cAMP receptor/Wiskott-Aldrich syndrome protein family verprolin-homologous (SCAR/WAVE)-like protein. We found that TUT1 contains conserved functional domains found in eukaryotic SCAR/WAVE proteins, and was able to activate Actin-related protein2/3 to promote actin nucleation and polymerization in vitro. Consistently, tut1 mutants show defects in the arrangement of actin filaments in trichome. These results indicate that TUT1 is a functional SCAR/WAVE protein and plays an important role in panicle development.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures





References
-
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005) Cytokinin oxidase regulates rice grain production. Science 309: 741–745 - PubMed
-
- Beltzner CC, Pollard TD (2008) Pathway of actin filament branch formation by Arp2/3 complex. J Biol Chem 283: 7135–7144 - PubMed
-
- Bird D, Beisson F, Brigham A, Shin J, Greer S, Jetter R, Kunst L, Wu X, Yephremov A, Samuels L (2007) Characterization of Arabidopsis ABCG11/WBC11, an ATP binding cassette (ABC) transporter that is required for cuticular lipid secretion. Plant J 52: 485–498 - PubMed
-
- Blanchoin L, Amann KJ, Higgs HN, Marchand JB, Kaiser DA, Pollard TD (2000) Direct observation of dendritic actin filament networks nucleated by Arp2/3 complex and WASP/Scar proteins. Nature 404: 1007–1011 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

