The developmental origins of the mammalian ovarian reserve
- PMID: 26243868
- PMCID: PMC4529035
- DOI: 10.1242/dev.125211
The developmental origins of the mammalian ovarian reserve
Abstract
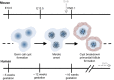
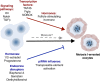
The adult mammalian ovary is devoid of definitive germline stem cells. As such, female reproductive senescence largely results from the depletion of a finite ovarian follicle pool that is produced during embryonic development. Remarkably, the crucial nature and regulation of follicle assembly and survival during embryogenesis is just coming into focus. This developmental pathway involves the coordination of meiotic progression and the breakdown of germ cell cysts into individual oocytes housed within primordial follicles. Recent evidence also indicates that genetic and environmental factors can specifically perturb primordial follicle assembly. Here, we review the cellular and molecular mechanisms by which the mammalian ovarian reserve is established, highlighting the presence of a crucial checkpoint that allows survival of only the highest-quality oocytes.
Keywords: Cyst breakdown; Meiosis; Oocyte; Ovary; Primordial follicle.
© 2015. Published by The Company of Biologists Ltd.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

