Expression of Opacity Proteins Interferes with the Transmigration of Neisseria gonorrhoeae across Polarized Epithelial Cells
- PMID: 26244560
- PMCID: PMC4526573
- DOI: 10.1371/journal.pone.0134342
Expression of Opacity Proteins Interferes with the Transmigration of Neisseria gonorrhoeae across Polarized Epithelial Cells
Abstract
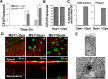
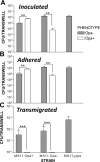
Neisseria gonorrhoeae (GC) establishes infection at the mucosal surface of the human genital tract, most of which is lined with polarized epithelial cells. GC can cause localized as well as disseminated infections, leading to various complications. GC constantly change their surface structures via phase and antigenic variation, which has been implicated as a means for GC to establish infection at various anatomic locations of male and female genital tracks. However, the exact contribution of each surface molecule to bacterial infectivity remains elusive due to their phase variation. Using a GC derivative that is genetically devoid of all opa genes (MS11∆Opa), this study shows that Opa expression interferes with GC transmigration across polarized human epithelial cells. MS11∆Opa transmigrates across polarized epithelial cells much faster and to a greater extent than MS11Opa+, while adhering at a similar level as MS11Opa+. When MS11Opa+, able to phase vary Opa expression, was inoculated, only those bacteria that turn off Opa expression transmigrate across the polarized epithelial monolayer. Similar to bacteria alone or co-cultured with non-polarized epithelial cells, MS11∆Opa fails to form large microcolonies at the apical surface of polarized epithelial cells. Apical inoculation of MS11Opa+, but not MS11∆Opa, induces the recruitment of the Opa host-cell receptor carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) to the apical junction and the vicinity of bacterial adherent sites. Our results suggest that Opa expression limits gonococcal ability to invade into subepithelial tissues by forming tight interactions with neighboring bacteria and by inducing CEACAM redistribution to cell junctions.
Conflict of interest statement
Figures



Similar articles
-
Specific Binding to Differentially Expressed Human Carcinoembryonic Antigen-Related Cell Adhesion Molecules Determines the Outcome of Neisseria gonorrhoeae Infections along the Female Reproductive Tract.Infect Immun. 2018 Jul 23;86(8):e00092-18. doi: 10.1128/IAI.00092-18. Print 2018 Aug. Infect Immun. 2018. PMID: 29760215 Free PMC article.
-
Escherichia coli expressing a Neisseria gonorrhoeae opacity-associated outer membrane protein invade human cervical and endometrial epithelial cell lines.Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5512-6. doi: 10.1073/pnas.89.12.5512. Proc Natl Acad Sci U S A. 1992. PMID: 1608963 Free PMC article.
-
Neisseria gonorrhoeae breaches the apical junction of polarized epithelial cells for transmigration by activating EGFR.Cell Microbiol. 2013 Jun;15(6):1042-57. doi: 10.1111/cmi.12099. Epub 2013 Jan 21. Cell Microbiol. 2013. PMID: 23279089 Free PMC article.
-
Opa proteins and CEACAMs: pathways of immune engagement for pathogenic Neisseria.FEMS Microbiol Rev. 2011 May;35(3):498-514. doi: 10.1111/j.1574-6976.2010.00260.x. Epub 2011 Jan 17. FEMS Microbiol Rev. 2011. PMID: 21204865 Review.
-
Interactions of pathogenic neisseriae with epithelial cell membranes.Annu Rev Cell Dev Biol. 2000;16:423-57. doi: 10.1146/annurev.cellbio.16.1.423. Annu Rev Cell Dev Biol. 2000. PMID: 11031243 Review.
Cited by
-
In Vitro Evaluation of Tellurium-Based AS101 Compound against Neisseria gonorrhoeae Infectivity.Microbiol Spectr. 2023 Mar 6;11(2):e0149622. doi: 10.1128/spectrum.01496-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36877078 Free PMC article.
-
Biomimetic Human Tissue Model for Long-Term Study of Neisseria gonorrhoeae Infection.Front Microbiol. 2019 Jul 31;10:1740. doi: 10.3389/fmicb.2019.01740. eCollection 2019. Front Microbiol. 2019. PMID: 31417529 Free PMC article.
-
Quantitative Examination of Antibiotic Susceptibility of Neisseria gonorrhoeae Aggregates Using ATP-utilization Commercial Assays and Live/Dead Staining.J Vis Exp. 2019 Feb 8;(144):10.3791/58978. doi: 10.3791/58978. J Vis Exp. 2019. PMID: 30799857 Free PMC article.
-
Phase-Variable Heptose I Glycan Extensions Modulate Efficacy of 2C7 Vaccine Antibody Directed against Neisseria gonorrhoeae Lipooligosaccharide.J Immunol. 2016 Jun 1;196(11):4576-86. doi: 10.4049/jimmunol.1600374. Epub 2016 May 2. J Immunol. 2016. PMID: 27183633 Free PMC article.
-
In Vitro Analysis of Matched Isolates from Localized and Disseminated Gonococcal Infections Suggests That Opa Expression Impacts Clinical Outcome.Pathogens. 2022 Feb 7;11(2):217. doi: 10.3390/pathogens11020217. Pathogens. 2022. PMID: 35215160 Free PMC article.
References
-
- Harvey HA, Jennings MP, Campbell CA, Williams R, Apicella MA. Receptor-mediated endocytosis of Neisseria gonorrhoeae into primary human urethral epithelial cells: the role of the asialoglycoprotein receptor. Mol Microbiol. 2001;42(3):659–72. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

