Retinal Conformation Changes Rhodopsin's Dynamic Ensemble
- PMID: 26244742
- PMCID: PMC4572577
- DOI: 10.1016/j.bpj.2015.06.046
Retinal Conformation Changes Rhodopsin's Dynamic Ensemble
Abstract
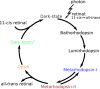
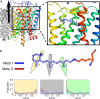
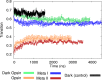
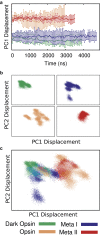
G protein-coupled receptors are vital membrane proteins that allosterically transduce biomolecular signals across the cell membrane. However, the process by which ligand binding induces protein conformation changes is not well understood biophysically. Rhodopsin, the mammalian dim-light receptor, is a unique test case for understanding these processes because of its switch-like activity; the ligand, retinal, is bound throughout the activation cycle, switching from inverse agonist to agonist after absorbing a photon. By contrast, the ligand-free opsin is outside the activation cycle and may behave differently. We find that retinal influences rhodopsin dynamics using an ensemble of all-atom molecular dynamics simulations that in aggregate contain 100 μs of sampling. Active retinal destabilizes the inactive state of the receptor, whereas the active ensemble was more structurally homogenous. By contrast, simulations of an active-like receptor without retinal present were much more heterogeneous than those containing retinal. These results suggest allosteric processes are more complicated than a ligand inducing protein conformational changes or simply capturing a shifted ensemble as outlined in classic models of allostery.
Copyright © 2015 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

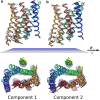
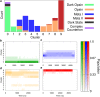
References
-
- Mombaerts P. Seven-transmembrane proteins as odorant and chemosensory receptors. Science. 1999;286:707–711. - PubMed
-
- Firestein S. The good taste of genomics. Nature. 2000;404:552–553. - PubMed
-
- Fredriksson R., Lagerström M.C., Schiöth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

