Safety, Pharmacokinetics and Efficacy of Dolutegravir in Treatment-experienced HIV-1 Infected Adolescents: Forty-eight-week Results from IMPAACT P1093
- PMID: 26244832
- PMCID: PMC4604048
- DOI: 10.1097/INF.0000000000000848
Safety, Pharmacokinetics and Efficacy of Dolutegravir in Treatment-experienced HIV-1 Infected Adolescents: Forty-eight-week Results from IMPAACT P1093
Abstract
Objective: To assess the pharmacokinetics (PK), safety and efficacy of dolutegravir plus optimized background regimen in HIV-infected treatment-experienced adolescents.
Methods: Children older than 12 to younger than 18 years received dolutegravir weight-based fixed doses at approximately 1.0 mg/kg once daily in a phase I/II multicenter open label 48-week study. Intensive PK evaluation was done at steady state after dolutegravir was added to a failing regimen or started at the end of a treatment interruption. Safety and HIV RNA and CD4 cell count assessments were performed through week 48.
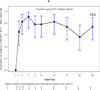
Results: Twenty-three adolescents were enrolled and 22 (96%) completed the 48-week study visit. Median age and weight were 15 years and 52 kg, respectively. Median [interquartile range (IQR)] baseline CD4+ cell count was 466 cells/μL (297, 771). Median (IQR) baseline HIV-1 RNA log10 was 4.3 log10 copies/mL (3.9, 4.6). Dolutegravir geometric mean of the area under the plasma concentration-time curve from time of administration to 24 hours after dosing (AUC0-24) and 24 hour postdose concentration (C24) were 46.0 μg hours/mL and 0.90 μg/mL, respectively, which were within the study targets based on adult PK ranges. Virologic success with an HIV RNA <400 copies/mL was achieved in 74% [95% confidence interval (CI): 52-90%] at week 48. Additionally, 61% (95% CI: 39-80%) had an HIV RNA <50 copies/mL at week 48. Median (IQR) gain in CD4 cell count at week 48 was 84 cells/μL (-81, 238). Dolutegravir was well tolerated, with no grade 4 adverse events, serious adverse events or discontinuations because of serious adverse events.
Conclusions: Dolutegravir achieved target PK exposures in adolescents. Dolutegravir was safe and well tolerated, providing good virologic efficacy through week 48.
Trial registration: ClinicalTrials.gov NCT01302847.
Conflict of interest statement
Figures


References
-
- Mocroft A, Ledergerber B, Katlama C, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003;362:22–29. - PubMed
-
- Gortmaker SL, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–1528. - PubMed
-
- Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:725–731. - PubMed
-
- Resino S, Resino R, Maria Bellon J, et al. Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clin Infect Dis. 2006;43:243–252. - PubMed
-
- Kourtis AP, Bansil P, Posner SF, Johnson C, Jamieson DJ. Trends in hospitalizations of HIV-infected children and adolescents in the United States: analysis of data from the 1994–2003 Nationwide Inpatient Sample. Pediatrics. 2007;120:e236–e243. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

