Francisella tularensis: No Evidence for Transovarial Transmission in the Tularemia Tick Vectors Dermacentor reticulatus and Ixodes ricinus
- PMID: 26244842
- PMCID: PMC4526560
- DOI: 10.1371/journal.pone.0133593
Francisella tularensis: No Evidence for Transovarial Transmission in the Tularemia Tick Vectors Dermacentor reticulatus and Ixodes ricinus
Abstract
Background: Tularemia is a zoonosis caused by the Francisella tularensis, a highly infectious Gram-negative coccobacillus. Due to easy dissemination, multiple routes of infection, high environmental contamination and morbidity and mortality rates, Francisella is considered a potential bioterrorism threat and classified as a category A select agent by the CDC. Tick bites are among the most prevalent modes of transmission, and ticks have been indicated as a possible reservoir, although their reservoir competence has yet to be defined. Tick-borne transmission of F. tularensis was recognized in 1923, and transstadial transmission has been demonstrated in several tick species. Studies on transovarial transmission, however, have reported conflicting results.
Objective: The aim of this study was to evaluate the role of ticks as reservoirs for Francisella, assessing the transovarial transmission of F. tularensis subsp. holarctica in ticks, using experimentally-infected females of Dermacentor reticulatus and Ixodes ricinus.
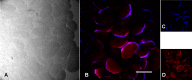
Results: Transmission electron microscopy and fluorescence in situ hybridization showed F. tularensis within oocytes. However, cultures and bioassays of eggs and larvae were negative; in addition, microscopy techniques revealed bacterial degeneration/death in the oocytes.
Conclusions: These results suggest that bacterial death might occur in oocytes, preventing the transovarial transmission of Francisella. We can speculate that Francisella does not have a defined reservoir, but that rather various biological niches (e.g. ticks, rodents), that allow the bacterium to persist in the environment. Our results, suggesting that ticks are not competent for the bacterium vertical transmission, are congruent with this view.
Conflict of interest statement
Figures





Similar articles
-
Occurrence of Francisella spp. in Dermacentor reticulatus and Ixodes ricinus ticks collected in eastern Poland.Ticks Tick Borne Dis. 2015 Apr;6(3):253-7. doi: 10.1016/j.ttbdis.2015.01.005. Epub 2015 Jan 31. Ticks Tick Borne Dis. 2015. PMID: 25666656
-
Presence of an emerging subclone of Francisella tularensis holarctica in Ixodes ricinus ticks from south-western Germany.Ticks Tick Borne Dis. 2013 Feb;4(1-2):93-100. doi: 10.1016/j.ttbdis.2012.09.001. Epub 2012 Nov 7. Ticks Tick Borne Dis. 2013. PMID: 23141103
-
Detection of a novel Francisella in Dermacentor reticulatus: a need for careful evaluation of PCR-based identification of Francisella tularensis in Eurasian ticks.Vector Borne Zoonotic Dis. 2009 Feb;9(1):123-6. doi: 10.1089/vbz.2008.0010. Epub 2008 Oct 22. Vector Borne Zoonotic Dis. 2009. PMID: 18945184
-
Ticks and Tularemia: Do We Know What We Don't Know?Front Cell Infect Microbiol. 2019 May 8;9:146. doi: 10.3389/fcimb.2019.00146. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31139576 Free PMC article. Review.
-
Genotyping of Francisella tularensis, the causative agent of tularemia.J AOAC Int. 2010 Nov-Dec;93(6):1930-43. J AOAC Int. 2010. PMID: 21313823 Review.
Cited by
-
The Tick-Borne Diseases STING study: Real-time PCR analysis of three emerging tick-borne pathogens in ticks that have bitten humans in different regions of Sweden and the Aland islands, Finland.Infect Ecol Epidemiol. 2019 Nov 2;9(1):1683935. doi: 10.1080/20008686.2019.1683935. eCollection 2019. Infect Ecol Epidemiol. 2019. PMID: 31741721 Free PMC article.
-
Disparate dynamics of pathogen prevalence in Ixodes ricinus and Dermacentor reticulatus ticks occurring sympatrically in diverse habitats.Sci Rep. 2023 Jun 30;13(1):10645. doi: 10.1038/s41598-023-37748-z. Sci Rep. 2023. PMID: 37391552 Free PMC article.
-
Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0.PLoS Biol. 2020 Jul 14;18(7):e3000411. doi: 10.1371/journal.pbio.3000411. eCollection 2020 Jul. PLoS Biol. 2020. PMID: 32663221 Free PMC article.
-
Ecology of Francisella tularensis.Annu Rev Entomol. 2020 Jan 7;65:351-372. doi: 10.1146/annurev-ento-011019-025134. Epub 2019 Oct 10. Annu Rev Entomol. 2020. PMID: 31600457 Free PMC article. Review.
-
Molecular investigation of Coxiella burnetii and Francisella tularensis infection in ticks in northern, western, and northwestern Iran.PLoS One. 2023 Aug 17;18(8):e0289567. doi: 10.1371/journal.pone.0289567. eCollection 2023. PLoS One. 2023. PMID: 37590254 Free PMC article.
References
-
- Friend M. Tularemia: Reston, Va, US Geological Survey, Circular 1297. 2006. p.1- 68.
-
- Reese SM, Petersen JM, Sheldon SW, Dolan MC, Dietrich G, Piesman J, et al. Transmission efficiency of Francisella tularensis by adult American dog ticks (Acari: Ixodidae). J Med Entomol. 2011; 48: 884–890. - PubMed
-
- Brown RN, Lane RS, Dennis DT. Geographic distribution of tick-borne diseases and their vectors In: Goodman JL, Dennis DT, Sonenshine DE, editor. Tick-borne diseases of humans. ASM Press, Washington, USA; 2005. pp. 363–391.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

