Likelihood of Null Effects of Large NHLBI Clinical Trials Has Increased over Time
- PMID: 26244868
- PMCID: PMC4526697
- DOI: 10.1371/journal.pone.0132382
Likelihood of Null Effects of Large NHLBI Clinical Trials Has Increased over Time
Abstract
Background: We explore whether the number of null results in large National Heart Lung, and Blood Institute (NHLBI) funded trials has increased over time.
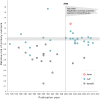
Methods: We identified all large NHLBI supported RCTs between 1970 and 2012 evaluating drugs or dietary supplements for the treatment or prevention of cardiovascular disease. Trials were included if direct costs >$500,000/year, participants were adult humans, and the primary outcome was cardiovascular risk, disease or death. The 55 trials meeting these criteria were coded for whether they were published prior to or after the year 2000, whether they registered in clinicaltrials.gov prior to publication, used active or placebo comparator, and whether or not the trial had industry co-sponsorship. We tabulated whether the study reported a positive, negative, or null result on the primary outcome variable and for total mortality.
Results: 17 of 30 studies (57%) published prior to 2000 showed a significant benefit of intervention on the primary outcome in comparison to only 2 among the 25 (8%) trials published after 2000 (χ2=12.2,df= 1, p=0.0005). There has been no change in the proportion of trials that compared treatment to placebo versus active comparator. Industry co-sponsorship was unrelated to the probability of reporting a significant benefit. Pre-registration in clinical trials.gov was strongly associated with the trend toward null findings.
Conclusions: The number NHLBI trials reporting positive results declined after the year 2000. Prospective declaration of outcomes in RCTs, and the adoption of transparent reporting standards, as required by clinicaltrials.gov, may have contributed to the trend toward null findings.
Conflict of interest statement
Figures

Comment in
-
Transparency rules lead to large fall in positive trial results, analysis finds.BMJ. 2015 Aug 9;351:h4304. doi: 10.1136/bmj.h4304. BMJ. 2015. PMID: 26261243 No abstract available.
References
-
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337: 867–872. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

