Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer
- PMID: 26244877
- PMCID: PMC4562797
- DOI: 10.1056/NEJMoa1503747
Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer
Abstract
Background: Androgen-deprivation therapy (ADT) has been the backbone of treatment for metastatic prostate cancer since the 1940s. We assessed whether concomitant treatment with ADT plus docetaxel would result in longer overall survival than that with ADT alone.
Methods: We assigned men with metastatic, hormone-sensitive prostate cancer to receive either ADT plus docetaxel (at a dose of 75 mg per square meter of body-surface area every 3 weeks for six cycles) or ADT alone. The primary objective was to test the hypothesis that the median overall survival would be 33.3% longer among patients receiving docetaxel added to ADT early during therapy than among patients receiving ADT alone.
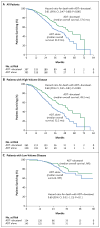
Results: A total of 790 patients (median age, 63 years) underwent randomization. After a median follow-up of 28.9 months, the median overall survival was 13.6 months longer with ADT plus docetaxel (combination therapy) than with ADT alone (57.6 months vs. 44.0 months; hazard ratio for death in the combination group, 0.61; 95% confidence interval [CI], 0.47 to 0.80; P<0.001). The median time to biochemical, symptomatic, or radiographic progression was 20.2 months in the combination group, as compared with 11.7 months in the ADT-alone group (hazard ratio, 0.61; 95% CI, 0.51 to 0.72; P<0.001). The rate of a prostate-specific antigen level of less than 0.2 ng per milliliter at 12 months was 27.7% in the combination group versus 16.8% in the ADT-alone group (P<0.001). In the combination group, the rate of grade 3 or 4 febrile neutropenia was 6.2%, the rate of grade 3 or 4 infection with neutropenia was 2.3%, and the rate of grade 3 sensory neuropathy and of grade 3 motor neuropathy was 0.5%.
Conclusions: Six cycles of docetaxel at the beginning of ADT for metastatic prostate cancer resulted in significantly longer overall survival than that with ADT alone. (Funded by the National Cancer Institute and others; ClinicalTrials.gov number, NCT00309985.).
Figures

Comment in
-
Adding chemotherapy to hormonal therapy prolongs survival in metastatic prostate cancer, study finds.BMJ. 2015 Aug 5;351:h4253. doi: 10.1136/bmj.h4253. BMJ. 2015. PMID: 26251343 No abstract available.
-
Prostate cancer: Docetaxel plus ADT significantly improves patient outcomes.Nat Rev Clin Oncol. 2015 Oct;12(10):563. doi: 10.1038/nrclinonc.2015.145. Epub 2015 Aug 25. Nat Rev Clin Oncol. 2015. PMID: 26305034 No abstract available.
-
Mapping the course after CHAARTED.Nat Rev Urol. 2015 Dec;12(12):656-8. doi: 10.1038/nrurol.2015.255. Epub 2015 Nov 3. Nat Rev Urol. 2015. PMID: 26526753 No abstract available.
-
CHAARTED/GETUG 12--docetaxel in non-castrate prostate cancers.Nat Rev Clin Oncol. 2015 Dec;12(12):687-8. doi: 10.1038/nrclinonc.2015.192. Epub 2015 Nov 10. Nat Rev Clin Oncol. 2015. PMID: 26552950 No abstract available.
-
Irrefutable evidence for the use of docetaxel in newly diagnosed metastatic prostate cancer: results from the STAMPEDE and CHAARTED trials.BMC Med. 2015 Dec 22;13:304. doi: 10.1186/s12916-015-0543-9. BMC Med. 2015. PMID: 26695172 Free PMC article.
-
Re: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer.J Urol. 2016 Jan;195(1):94. doi: 10.1016/j.juro.2015.10.055. Epub 2015 Oct 23. J Urol. 2016. PMID: 26699961 No abstract available.
-
Chemohormonal Therapy in Hormone-Sensitive Prostate Cancer.N Engl J Med. 2016 Jan 21;374(3):287. doi: 10.1056/NEJMc1511800. N Engl J Med. 2016. PMID: 26789883 No abstract available.
-
Chemohormonal Therapy in Hormone-Sensitive Prostate Cancer.N Engl J Med. 2016 Jan 21;374(3):286. doi: 10.1056/NEJMc1511800. N Engl J Med. 2016. PMID: 26789884 No abstract available.
-
Words of Wisdom. Re: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer.Eur Urol. 2016 Mar;69(3):540. doi: 10.1016/j.eururo.2015.12.027. Eur Urol. 2016. PMID: 26867727 No abstract available.
-
Words of Wisdom. Re: Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer.Eur Urol. 2016 Apr;69(4):755-6. doi: 10.1016/j.eururo.2016.01.020. Epub 2016 Feb 18. Eur Urol. 2016. PMID: 26972499 No abstract available.
-
Words of Wisdom: Re: Chemohormonal Therapy in Metastatic Hormone-sensitive Prostate Cancer.Eur Urol. 2016 Jan;69(1):178. doi: 10.1016/j.eururo.2015.10.037. Eur Urol. 2016. PMID: 27099882 No abstract available.
References
-
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–7. - PubMed
-
- Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet. 2000;355:1491–8. - PubMed
-
- Samson DJ, Seidenfeld J, Schmitt B, et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer. 2002;95:361–76. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA189829/CA/NCI NIH HHS/United States
- CA180795/CA/NCI NIH HHS/United States
- U10 CA180795/CA/NCI NIH HHS/United States
- U10 CA066636/CA/NCI NIH HHS/United States
- U10 CA086802/CA/NCI NIH HHS/United States
- U10 CA037403/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- CA180853/CA/NCI NIH HHS/United States
- CA180794/CA/NCI NIH HHS/United States
- U10 CA180799/CA/NCI NIH HHS/United States
- CA180799/CA/NCI NIH HHS/United States
- U10 CA180802/CA/NCI NIH HHS/United States
- CA189829/CA/NCI NIH HHS/United States
- U01 CA080098/CA/NCI NIH HHS/United States
- U10 CA021115/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- CA180820/CA/NCI NIH HHS/United States
- CA180802/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180801/CA/NCI NIH HHS/United States
- UG1 CA189828/CA/NCI NIH HHS/United States
- CA180801/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA023318/CA/NCI NIH HHS/United States
- CA180790/CA/NCI NIH HHS/United States
- CA180888/CA/NCI NIH HHS/United States
- U10 CA180794/CA/NCI NIH HHS/United States
- CA180821/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- CA31946/CA/NCI NIH HHS/United States
- U10 CA180853/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
