Multivalent Pneumococcal Protein Vaccines Comprising Pneumolysoid with Epitopes/Fragments of CbpA and/or PspA Elicit Strong and Broad Protection
- PMID: 26245351
- PMCID: PMC4580740
- DOI: 10.1128/CVI.00293-15
Multivalent Pneumococcal Protein Vaccines Comprising Pneumolysoid with Epitopes/Fragments of CbpA and/or PspA Elicit Strong and Broad Protection
Abstract
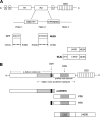
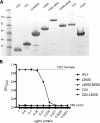
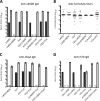
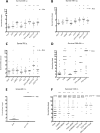
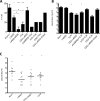
Immunization with the pneumococcal proteins pneumolysin (Ply), choline binding protein A (CbpA), or pneumococcal surface protein A (PspA) elicits protective responses against invasive pneumococcal disease in animal models. In this study, we used different mouse models to test the efficacy of a variety of multivalent protein-based vaccines that comprised various combinations of full-length or peptide regions of the immunogens Ply, CbpA, or PspA: Ply toxoid with the L460D substitution (referred to herein as L460D); L460D fused with protective peptide epitopes from CbpA (YPT-L460D-NEEK [YLN]); L460D fused with the CD2 peptide containing the proline-rich region (PRR) of PspA (CD2-L460D); a combination of L460D and H70 (L460D+H70), a slightly larger PspA-derived peptide containing the PRR and the SM1 region; H70+YLN; and other combinations. Each mouse was immunized either intraperitoneally (i.p.) or subcutaneously (s.c.) with three doses (at 2-week intervals) of the various antigen combinations in alum adjuvant and then challenged in mouse models featuring different infection routes with multiple Streptococcus pneumoniae strains. In the i.p. infection sepsis model, H70+YLN consistently provided significant protection against three different challenge strains (serotypes 1, 2, and 6A); the CD2+YLN and H70+L460D combinations also elicited significant protection. Protection against intravenous (i.v.) sepsis (type 3 and 6A challenge strains) was largely dependent on PspA-derived antigen components, and the most protection was elicited by H70 with or without L460D or YLN. In a type 4 intratracheal (i.t.) challenge model that results in progression to meningitis, antigen combinations that contained YLN elicited the strongest protection. Thus, the trivalent antigen combination of H70+YLN elicited the strongest and broadest protection in diverse pneumococcal challenge models.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
References
-
- O'Brien KL, Wolfson LJ, Watt JP, Henkle E, Deloria-Knoll M, McCall N, Lee E, Mulholland K, Levine OS, Cherian T, Hib and Pneumococcal Global Burden of Disease Study Team. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902. doi:10.1016/S0140-6736(09)61204-6. - DOI - PubMed
-
- World Health Organization. 2008. 23-valent pneumococcal polysaccharide vaccine. WHO position paper. Wkly Epidemiol Rec 83:373–384. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

