Evaluation of the Specificity of Two Enzyme Immunoassays for Coccidioidomycosis by Using Sera from a Region of Endemicity and a Region of Nonendemicity
- PMID: 26245352
- PMCID: PMC4580743
- DOI: 10.1128/CVI.00375-15
Evaluation of the Specificity of Two Enzyme Immunoassays for Coccidioidomycosis by Using Sera from a Region of Endemicity and a Region of Nonendemicity
Abstract
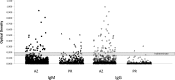
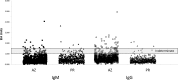
Coccidioidomycosis (CM), a serious life-threatening fungal infection endemic to arid regions of the western United States and Mexico, can be challenging to diagnose in a timely manner. Commercially developed enzyme immunoassays (EIAs) (from Meridian Biosciences and Immuno-Mycologics [IMMY]) have provided faster, simpler means for serodiagnosis; however, independent evaluations have questioned EIA specificity, particularly IgM-positive/IgG-negative results. This study was conducted to evaluate EIA specificity among persons residing in Puerto Rico (n = 534), where CM is not endemic (who were not likely to have been exposed to Coccidioides spp.), compared to blood bank donors residing in Arizona (n = 1,218), where CM is endemic. Upon comparing serum reactivity between Puerto Rico and Arizona, the Meridian EIA showed a significant difference in IgG reactivity (0.37% versus 3.6%; P < 0.001) but not IgM reactivity (3.4% versus 2.4%; P = 0.31). No IgM-/IgG-reactive sera were detected among sera from Puerto Rico, compared to 7 (0.57%) sera from Arizona. Similar results were observed using the IMMY EIA, although significantly (P = 0.03) fewer IgM-reactive sera from Arizona were observed, compared to the Meridian EIA. EIA-reactive sera were also evaluated by immunodiffusion before and after 3- to 4-fold concentration of the sera. These results demonstrate that elevated IgG EIA reactivity is present in sera from healthy individuals in regions of endemicity and that IgM EIA reactivity observed in sera from individuals residing outside regions of endemicity is most likely nonspecific. Other criteria, including clinical and microbiological evaluations, should be taken into account when interpreting results from surveillance studies and other reporting measures.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Interlaboratory agreement of coccidioidomycosis enzyme immunoassay from two different manufacturers.Med Mycol. 2019 Jun 1;57(4):441-446. doi: 10.1093/mmy/myy059. Med Mycol. 2019. PMID: 30085141
-
Comparison of three anti-coccidioides antibody enzyme immunoassay kits for the diagnosis of coccidioidomycosis.Med Mycol. 2020 Aug 1;58(6):774-778. doi: 10.1093/mmy/myz125. Med Mycol. 2020. PMID: 32277825
-
Clinical Laboratory Utility of a Humanized Antibody in Commercially Available Enzyme Immunoassays for Coccidioidomycosis.Microbiol Spectr. 2022 Oct 26;10(5):e0257322. doi: 10.1128/spectrum.02573-22. Epub 2022 Sep 19. Microbiol Spectr. 2022. PMID: 36121238 Free PMC article.
-
Laboratory aspects in the diagnosis of coccidioidomycosis.Ann N Y Acad Sci. 2007 Sep;1111:301-14. doi: 10.1196/annals.1406.049. Epub 2007 Mar 15. Ann N Y Acad Sci. 2007. PMID: 17363434 Review.
-
Serology of coccidioidomycosis.Clin Microbiol Rev. 1990 Jul;3(3):247-68. doi: 10.1128/CMR.3.3.247. Clin Microbiol Rev. 1990. PMID: 2200605 Free PMC article. Review.
Cited by
-
Development of an enzyme immunoassay for detection of antibodies against Coccidioides in dogs and other mammalian species.PLoS One. 2017 Apr 5;12(4):e0175081. doi: 10.1371/journal.pone.0175081. eCollection 2017. PLoS One. 2017. PMID: 28380017 Free PMC article.
-
Development of a Quantitative Antigen Assay to Detect Coccidioidal Chitinase-1 (CTS1) in Human Serum.Open Forum Infect Dis. 2021 Jun 28;8(7):ofab344. doi: 10.1093/ofid/ofab344. eCollection 2021 Jul. Open Forum Infect Dis. 2021. PMID: 34337097 Free PMC article.
-
Microbiological Laboratory Testing in the Diagnosis of Fungal Infections in Pulmonary and Critical Care Practice. An Official American Thoracic Society Clinical Practice Guideline.Am J Respir Crit Care Med. 2019 Sep 1;200(5):535-550. doi: 10.1164/rccm.201906-1185ST. Am J Respir Crit Care Med. 2019. PMID: 31469325 Free PMC article.
-
Characterization of an Uncinocarpus reesii-expressed recombinant tube precipitin antigen of Coccidioides posadasii for serodiagnosis.PLoS One. 2019 Aug 14;14(8):e0221228. doi: 10.1371/journal.pone.0221228. eCollection 2019. PLoS One. 2019. PMID: 31412087 Free PMC article. Clinical Trial.
-
Top Questions in the Diagnosis and Treatment of Coccidioidomycosis.Open Forum Infect Dis. 2017 Sep 12;4(4):ofx197. doi: 10.1093/ofid/ofx197. eCollection 2017 Fall. Open Forum Infect Dis. 2017. PMID: 29670928 Free PMC article.
References
-
- Nguyen C, Barker BM, Hoover S, Nix DE, Ampel NM, Frelinger JA, Orbach MJ, Galgiani JN. 2013. Recent advances in our understanding of the environmental, epidemiological, immunological, and clinical dimensions of coccidioidomycosis. Clin Microbiol Rev 26:505–525. doi:10.1128/CMR.00005-13. - DOI - PMC - PubMed
-
- Thompson G III, Stevens D, Clemons K, Fierer J, Johnson R, Sykes J, Rutherford G, Peterson M, Taylor J, Chaturvedi V. 2015. Call for a California coccidioidomycosis consortium to face the top ten challenges posed by a recalcitrant regional disease. Mycopathologia 179:1–9. doi:10.1007/s11046-014-9816-7. - DOI - PubMed
-
- Zartarian M, Peterson EM, de la Maza LM. 1997. Detection of antibodies to Coccidioides immitis by enzyme immunoassay. Am J Clin Pathol 107:148–153. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

