Glutamate Receptors in the Central Nucleus of the Amygdala Mediate Cisplatin-Induced Malaise and Energy Balance Dysregulation through Direct Hindbrain Projections
- PMID: 26245970
- PMCID: PMC4524978
- DOI: 10.1523/JNEUROSCI.0440-15.2015
Glutamate Receptors in the Central Nucleus of the Amygdala Mediate Cisplatin-Induced Malaise and Energy Balance Dysregulation through Direct Hindbrain Projections
Abstract
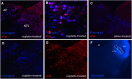
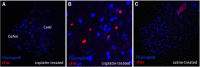
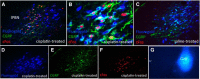
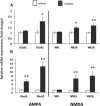
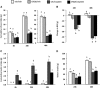
Cisplatin chemotherapy is used commonly to treat a variety of cancers despite severe side effects such as nausea, vomiting, and anorexia that compromise quality of life and limit treatment adherence. The neural mechanisms mediating these side effects remain elusive despite decades of clinical use. Recent data highlight the dorsal vagal complex (DVC), lateral parabrachial nucleus (lPBN), and central nucleus of the amygdala (CeA) as potential sites of action in mediating the side effects of cisplatin. Here, results from immunohistochemical studies in rats identified a population of cisplatin-activated DVC neurons that project to the lPBN and a population of cisplatin-activated lPBN calcitonin gene-related peptide (CGRP, a marker for glutamatergic neurons in the lPBN) neurons that project to the CeA, outlining a neuroanatomical circuit that is activated by cisplatin. CeA gene expressions of AMPA and NMDA glutamate receptor subunits were markedly increased after cisplatin treatment, suggesting that CeA glutamate receptor signaling plays a role in mediating cisplatin side effects. Consistent with gene expression results, behavioral/pharmacological data showed that CeA AMPA/kainate receptor blockade attenuates cisplatin-induced pica (a proxy for nausea/behavioral malaise in nonvomiting laboratory rodents) and that CeA NMDA receptor blockade attenuates cisplatin-induced anorexia and body weight loss in addition to pica, demonstrating that glutamate receptor signaling in the CeA is critical for the energy balance dysregulation caused by cisplatin treatment. Together, these data highlight a novel circuit and CGRP/glutamatergic mechanism through which cisplatin-induced malaise and energy balance dysregulation are mediated.
Significance statement: To treat cancer effectively, patients must follow prescribed chemotherapy treatments without interruption, yet most cancer treatments produce side effects that devastate quality of life (e.g., nausea, vomiting, anorexia, weight loss). Although hundreds of thousands of patients undergo chemotherapies each year, the neural mechanisms mediating their side effects are unknown. The current data outline a neural circuit activated by cisplatin chemotherapy and demonstrate that glutamate signaling in the amygdala, arising from hindbrain projections, is required for the full expression of cisplatin-induced malaise, anorexia, and body weight loss. Together, these data help to characterize the neural circuits and neurotransmitters mediating chemotherapy-induced energy balance dysregulation, which will ultimately provide an opportunity for the development of well tolerated cancer and anti-emetic treatments.
Keywords: amygdala; anorexia; cisplatin; dorsal vagal complex; glutamate; parabrachial nucleus.
Copyright © 2015 the authors 0270-6474/15/3511094-11$15.00/0.
Figures


References
-
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L, LEAD-6 Study Group Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
