Anion homeostasis is important for non-lytic release of BK polyomavirus from infected cells
- PMID: 26246492
- PMCID: PMC4554916
- DOI: 10.1098/rsob.150041
Anion homeostasis is important for non-lytic release of BK polyomavirus from infected cells
Abstract
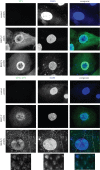
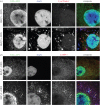
BK polyomavirus (BKPyV) is a member of a family of potentially oncogenic viruses, whose reactivation can cause severe pathological conditions in transplant patients, leading to graft rejection. As with many non-enveloped viruses, it is assumed that virus release occurs through lysis of the host cell. We now show the first evidence for a non-lytic release pathway for BKPyV and that this pathway can be blocked by the anion channel inhibitor DIDS. Our data show a dose-dependent effect of DIDS on the release of BKPyV virions. We also observed an accumulation of viral capsids in large LAMP-1-positive acidic organelles within the cytoplasm of cells upon DIDS treatment, suggesting potential late endosome or lysosome-related compartments are involved in non-lytic BKPyV release. These data highlight a novel mechanism by which polyomaviruses can be released from infected cells in an active and non-lytic manner, and that anion homeostasis regulation is important in this pathway.
Keywords: BKPyV; DIDS; anion homeostasis; polyomavirus; virus release.
© 2015 The Authors.
Figures



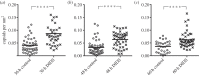
References
-
- Gardner SD, Field AM, Coleman DV, Hulme B. 1971. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet 1, 1253–1257. (doi:10.1016/S0140-6736(71)91776-4) - DOI - PubMed
-
- Padgett BL, Walker DL, ZuRhein GM, Eckroade RJ, Dessel BH. 1971. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet 1, 1257–1260. (doi:10.1016/S0140-6736(71)91777-6) - DOI - PubMed
-
- Feng H, Shuda M, Chang Y, Moore PS. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319, 1096–1100. (doi:10.1126/science.1152586) - DOI - PMC - PubMed
-
- Buck CB, Lowy DR. 2009. Getting stronger: the relationship between a newly identified virus and Merkel cell carcinoma. J. Investig. Dermatol. 129, 9–11. (doi:10.1038/jid.2008.302) - DOI - PMC - PubMed
-
- Johne R, Buck CB, Allander T, Atwood WJ, Garcea RL, Imperiale MJ, Major EO, Ramqvist T, Norkin LC. 2011. Taxonomical developments in the family Polyomaviridae. Arch. Virol. 156, 1627–1634. (doi:10.1007/s00705-011-1008-x) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
