A Druggable Pocket at the Nucleocapsid/Phosphoprotein Interaction Site of Human Respiratory Syncytial Virus
- PMID: 26246564
- PMCID: PMC4621127
- DOI: 10.1128/JVI.01612-15
A Druggable Pocket at the Nucleocapsid/Phosphoprotein Interaction Site of Human Respiratory Syncytial Virus
Abstract
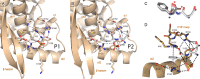
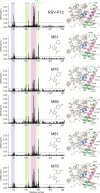
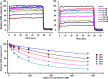
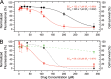
Presently, respiratory syncytial virus (RSV), the main cause of severe respiratory infections in infants, cannot be treated efficiently with antivirals. However, its RNA-dependent polymerase complex offers potential targets for RSV-specific drugs. This includes the recognition of its template, the ribonucleoprotein complex (RNP), consisting of genomic RNA encapsidated by the RSV nucleoprotein, N. This recognition proceeds via interaction between the phosphoprotein P, which is the main polymerase cofactor, and N. The determinant role of the C terminus of P, and more particularly of the last residue, F241, in RNP binding and viral RNA synthesis has been assessed previously. Here, we provide detailed structural insight into this crucial interaction for RSV polymerase activity. We solved the crystallographic structures of complexes between the N-terminal domain of N (N-NTD) and C-terminal peptides of P and characterized binding by biophysical approaches. Our results provide a rationale for the pivotal role of F241, which inserts into a well-defined N-NTD pocket. This primary binding site is completed by transient contacts with upstream P residues outside the pocket. Based on the structural information of the N-NTD:P complex, we identified inhibitors of this interaction, selected by in silico screening of small compounds, that efficiently bind to N and compete with P in vitro. One of the compounds displayed inhibitory activity on RSV replication, thereby strengthening the relevance of N-NTD for structure-based design of RSV-specific antivirals.
Importance: Respiratory syncytial virus (RSV) is a widespread pathogen that is a leading cause of acute lower respiratory infections in infants worldwide. RSV cannot be treated efficiently with antivirals, and no vaccine is presently available, with the development of pediatric vaccines being particularly challenging. Therefore, there is a need for new therapeutic strategies that specifically target RSV. The interaction between the RSV phosphoprotein P and the ribonucleoprotein complex is critical for viral replication. In this study, we identified the main structural determinants of this interaction, and we used them to screen potential inhibitors in silico. We found a family of molecules that were efficient competitors of P in vitro and showed inhibitory activity on RSV replication in cellular assays. These compounds provide a basis for a pharmacophore model that must be improved but that holds promises for the design of new RSV-specific antivirals.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




Similar articles
-
Interactions between the Nucleoprotein and the Phosphoprotein of Pneumoviruses: Structural Insight for Rational Design of Antivirals.Viruses. 2021 Dec 6;13(12):2449. doi: 10.3390/v13122449. Viruses. 2021. PMID: 34960719 Free PMC article. Review.
-
Identification and characterization of the binding site of the respiratory syncytial virus phosphoprotein to RNA-free nucleoprotein.J Virol. 2015 Apr;89(7):3484-96. doi: 10.1128/JVI.03666-14. Epub 2015 Jan 7. J Virol. 2015. PMID: 25568210 Free PMC article.
-
Fine mapping and characterization of the L-polymerase-binding domain of the respiratory syncytial virus phosphoprotein.J Virol. 2015 Apr;89(8):4421-33. doi: 10.1128/JVI.03619-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653447 Free PMC article.
-
Structural modeling, energetic analysis and molecular design of a π-stacking system at the complex interface of pediatric respiratory syncytial virus nucleocapsid with the C-terminal peptide of phosphoprotein.Biophys Chem. 2023 Jan;292:106916. doi: 10.1016/j.bpc.2022.106916. Epub 2022 Oct 29. Biophys Chem. 2023. PMID: 36343393
-
Structural Insights into the Respiratory Syncytial Virus RNA Synthesis Complexes.Viruses. 2021 May 5;13(5):834. doi: 10.3390/v13050834. Viruses. 2021. PMID: 34063087 Free PMC article. Review.
Cited by
-
Interactions between the Nucleoprotein and the Phosphoprotein of Pneumoviruses: Structural Insight for Rational Design of Antivirals.Viruses. 2021 Dec 6;13(12):2449. doi: 10.3390/v13122449. Viruses. 2021. PMID: 34960719 Free PMC article. Review.
-
Docking Screens for Novel Ligands Conferring New Biology.J Med Chem. 2016 May 12;59(9):4103-20. doi: 10.1021/acs.jmedchem.5b02008. Epub 2016 Mar 15. J Med Chem. 2016. PMID: 26913380 Free PMC article. Review.
-
New Insights into Structural Disorder in Human Respiratory Syncytial Virus Phosphoprotein and Implications for Binding of Protein Partners.J Biol Chem. 2017 Feb 10;292(6):2120-2131. doi: 10.1074/jbc.M116.765958. Epub 2016 Dec 28. J Biol Chem. 2017. PMID: 28031463 Free PMC article.
-
EDP-938 Has a High Barrier to Resistance in Healthy Adults Experimentally Infected with Respiratory Syncytial Virus.J Infect Dis. 2025 Feb 20;231(2):e290-e298. doi: 10.1093/infdis/jiae471. J Infect Dis. 2025. PMID: 39441691 Free PMC article. Clinical Trial.
-
Structural landscape of the respiratory syncytial virus nucleocapsids.Nat Commun. 2023 Sep 15;14(1):5732. doi: 10.1038/s41467-023-41439-8. Nat Commun. 2023. PMID: 37714861 Free PMC article.
References
-
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555. doi:10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. 1995. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci U S A 92:11563–11567. doi:10.1073/pnas.92.25.11563. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

