Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity
- PMID: 26246566
- PMCID: PMC4580201
- DOI: 10.1128/JVI.01653-15
Murine Antibody Responses to Cleaved Soluble HIV-1 Envelope Trimers Are Highly Restricted in Specificity
Abstract
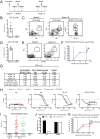
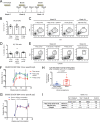
Generating neutralizing antibodies (nAbs) is a major goal of many current HIV-1 vaccine efforts. To be of practical value, these nAbs must be both potent and cross-reactive in order to be capable of preventing the transmission of the highly diverse and generally neutralization resistant (Tier-2) HIV-1 strains that are in circulation. The HIV-1 envelope glycoprotein (Env) spike is the only target for nAbs. To explore whether Tier-2 nAbs can be induced by Env proteins, we immunized conventional mice with soluble BG505 SOSIP.664 trimers that mimic the native Env spike. Here, we report that it is extremely difficult for murine B cells to recognize the Env epitopes necessary for inducing Tier-2 nAbs. Thus, while trimer-immunized mice raised Env-binding IgG Abs and had high-quality T follicular helper (Tfh) cell and germinal center (GC) responses, they did not make BG505.T332N nAbs. Epitope mapping studies showed that Ab responses in mice were specific to areas near the base of the soluble trimer. These areas are not well shielded by glycans and likely are occluded on virions, which is consistent with the lack of BG505.T332N nAbs. These data inform immunogen design and suggest that it is useful to obscure nonneutralizing epitopes presented on the base of soluble Env trimers and that the glycan shield of well-formed HIV Env trimers is virtually impenetrable for murine B cell receptors (BCRs).
Importance: Human HIV vaccine efficacy trials have not generated meaningful neutralizing antibodies to circulating HIV strains. One possible hindrance has been the lack of immunogens that properly mimic the native conformation of the HIV envelope trimer protein. Here, we tested the first generation of soluble, native-like envelope trimer immunogens in a conventional mouse model. We attempted to generate neutralizing antibodies to neutralization-resistant circulating HIV strains. Various vaccine strategies failed to induce neutralizing antibodies to a neutralization-resistant HIV strain. Further analysis revealed that mouse antibodies targeted areas near the bottom of the soluble envelope trimers. These areas are not easily accessible on the HIV virion due to occlusion by the viral membrane and may have resulted from an absence of glycan shielding. Our results suggest that obscuring the bottom of soluble envelope trimers is a useful strategy to reduce antibody responses to epitopes that are not useful for virus neutralization.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol Principal Investigators Group, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi:10.1126/science.1178746. - DOI - PMC - PubMed
-
- Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O'Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi:10.1126/science.1187659. - DOI - PMC - PubMed
-
- Falkowska E, Ramos A, Feng Y, Zhou T, Moquin S, Walker LM, Wu X, Seaman MS, Wrin T, Kwong PD, Wyatt RT, Mascola JR, Poignard P, Burton DR. 2012. PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. J Virol 86:4394–4403. doi:10.1128/JVI.06973-11. - DOI - PMC - PubMed
-
- Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O'Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang Z-Y, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, Comparative Sequencing Program NISC, Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602. doi:10.1126/science.1207532. - DOI - PMC - PubMed
-
- Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Georgiev IS, Chuang G-Y, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, la Peña de AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515:138–142. doi:10.1038/nature13601. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

